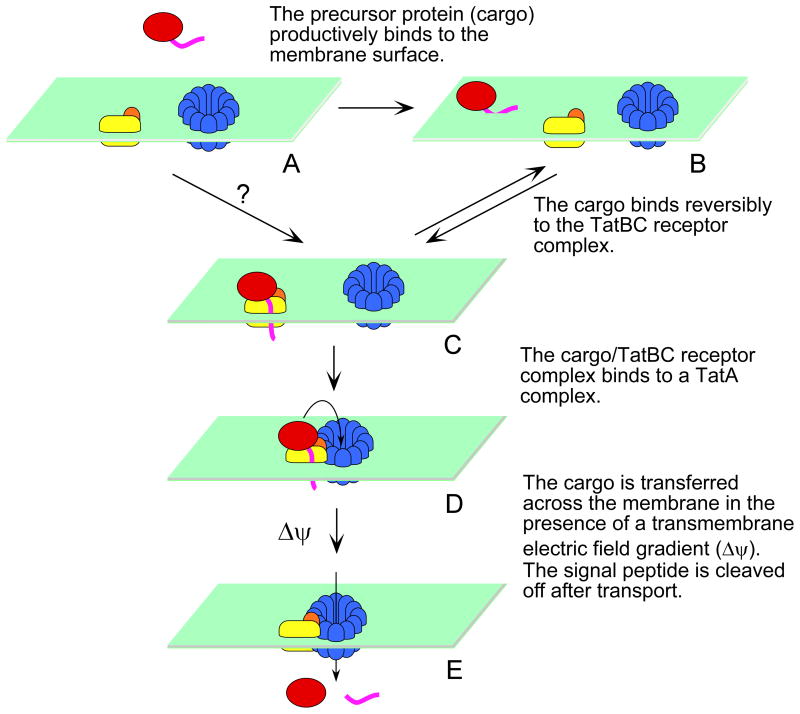
Figure 9. Model of E. coli Tat transport.
TatB (orange) and TatC (yellow) form a receptor complex, and TatA (blue) forms ring-like oligomers in resting membranes (A). The precursor protein (red) binds to the membrane surface (B) in addition to its binding site on the TatBC complex (C). The cargo/TatBC complex associates with an oligomerized TatA complex (D). Cargo is transported across the membrane in the presence of a transmembrane electric field gradient (Δψ). Signal peptide (magneta) cleavage occurs after transport of the precursor protein (E). We reported earlier that there are two ψ-dependent steps (Bageshwar & Musser, 2007). The first Δψ-dependent step can occur in the absence of precursor protein (not shown). The second Δψ-dependent step occurs after the precursor protein binds to the membrane, suggested here as the D → E step. For clarity, the likely possibility that the TatBC complexes form higher order oligomers is not shown. The fate of the signal peptide after transport is not known.