Abstract
Background
Pancreatic pseudocysts are a common complication of acute and chronic pancreatitis. They are diagnosed with imaging studies and can be treated successfully with a variety of methods: endoscopic transpapillary or transmural drainage, percutaneous catheter drainage, laparoscopic surgery, or open pseudocystoenterostomy.
Methods
Relevant publications that appeared from 1975 to 2008 were retrieved from the MEDLINE, PubMed and EMBASE databases for this review.
Results
Endoscopic pseudocyst drainage has a high success rate (79.2%) and a low complication rate (12.9%). Percutaneous drainage is mainly used for the emergency treatment of infected pancreatic pseudocysts. Open internal drainage and pseudocyst resection are surgical techniques with high success rates (>92%), but also higher morbidity (16%) and mortality (2.5%) than endoscopic treatment (mortality 0.7%). Laparoscopic pseudocystoenterostomy, a recently introduced procedure, is probably similar to the endoscopic techniques with regard to morbidity and mortality.
Conclusions
An interdisciplinary approach is best suited for the safe and effective stage-specific treatment of pancreatic pseudocysts. The different interventional techniques that are currently available have yet to be compared directly in randomized trials.
Keywords: pancreatitis, drainage, endoscopy, minimally invasive therapy, spontaneous remission
The decision whether to treat a patient with a pancreatic pseudocyst, as well as when and with what technique, is a difficult one. This review article is intended to help physicians base their therapeutic decisions on the current state of therapeutic technology and published data.
Definition
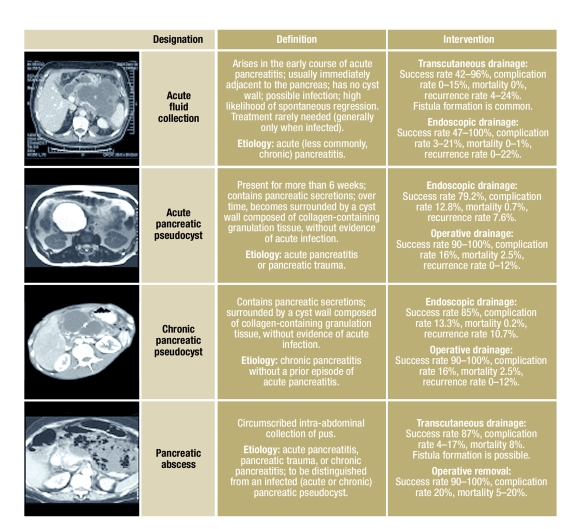
The management of cystic changes of the pancreas is an old problem. At the beginning of the twentieth century, Eugene Opie was the first to distinguish true pancreatic cysts, which are, by definition, lined by epithelium, from pseudocysts, which are surrounded by a wall composed of collagen and granulation tissue. The Atlanta classification of 1993 further distinguishes acute from chronic pseudocysts; the definition of chronicity is based, not on the age of the cyst, but rather on the underlying illness (figure 1) (1). The Atlanta classification consists of four distinct disease entities:
Figure 1.
The Atlanta classification and its therapeutic implications
acute fluid collections that develop early in the course of acute pancreatitis and do not yet have a cyst wall;
acute pancreatic pseudocysts, which arise as sequelae of acute pancreatitis or trauma, and whose wall consists of granulation tissue and extracellular matrix;
chronic pancreatic pseudocysts, which arise as sequelae of chronic pancreatitis and are likewise surrounded by a wall; and
pancreatic abscesses, which are intra-abdominal collections of pus immediately adjacent to the pancreas, without any large areas of necrosis.
Acute fluid collections, pancreatic pseudocysts, and pancreatic abscesses can be distinguished from one another by the history, imaging studies of the wall of the abnormality and its contents, and, if necessary, a needle aspiration of the content.
Incidence and etiology
Pancreatic pseudocysts often arise as a complication of acute or chronic pancreatitis. The prevalence of pancreatic pseudocysts in acute pancreatitis has been reported to range from 6% to 18.5% (2, 3). The prevalence of pancreatic pseudocysts in chronic pancreatitis is 20% to 40% (4). Pancreatic pseudocysts most commonly arise in patients with alcoholic chronic pancreatitis (70% to 78%) (5). The second most common cause is idiopathic chronic pancreatitis (6% to 16%), followed by biliary pancreatitis (6% to 8%).
Diagnostic evaluation
The diagnosis of a pancreatic pseudocyst is usually established by imaging studies, among which transabdominal ultrasonography is important as an initial investigation (7, e50, e51). Computerized tomography (CT) is often the imaging method of choice, with 82% to 100% sensitivity and 98% specificity (8). For the distinction of acute fluid collections from pancreatic abscesses and acute pancreatic pseudocysts, endosonography (EUS) has the highest sensitivity (93% to 100%) and specificity (92% to 98%) (e52). The diagnostic puncture of a pseudocyst under EUS guidance helps distinguish cystic malignancies from pseudocysts. A malignant lesion is more likely when the carcinoembryogenic antigen (CEA) value exceeds 192 ng/mL and when the cyst contents are highly viscous (e53) (9).
Course
The most important question in everyday clinical practice is often whether and when an acute or chronic pancreatic pseudocyst ought to be treated. In earlier studies on the clinical course of pancreatic pseudocysts, the rate of spontaneous regression ranged from 8% to 70%. Two major factors affecting the rate of regression are the size of the pseudocyst and the time that has elapsed since it was diagnosed. Bradley followed the course of 93 patients (10) and found that 42% of the acute pseudocysts that were less than six weeks old regressed without any intervention, as compared to only 8% of those that had been present for more than seven weeks.
In 1997, Gouyon was able to show convincingly in a multivariate analysis that the single independent parameter affecting the spontaneous regression of chronic pancreatic pseudocysts is the size of the cyst. Cysts under 4 cm in size regressed spontaneously significantly more frequently and had a lower rate of complications (11).
Maringhini and colleagues found that even acute pancreatic pseudocysts larger than 4 cm in size can still regress spontaneously in 65% of cases (3).
At least five studies document the fact that the great majority of acute pancreatic pseudocysts measuring less than 4 cm in diameter regress spontaneously and thus require no treatment, as long as they are asymptomatic.
The situation is different for chronic pancreatic pseudocysts. The rate of spontaneous regression in most studies is under 10%.
Other factors that make spontaneous regression unlikely include the presence of multiple cysts (e3), location in the tail of the pancreas (3), a wall thickness greater than 1 cm (12), lack of communication with Wirsung’s duct (13), proximal ductal stenosis, biliary or traumatic etiology, or an increase in size on follow-up (e3) (table 1).
Table 1. Spontaneous regression of pancreatic pseudocysts.
| Reference | Study period | Etiology alcohol | Diagnostic criteria (CT, US) | Chronic pancreatitis (ERCP) | Definition of pseudocysts by the Atlanta criteria | Spontaneous regression |
| Czaja et al. 1975 (e10) | 1970–1972 | 80% | No | No | No | 70% |
| Pollak et al. 1978 (e2) | 1966–1976 | NA | Some | No | No | 30% |
| McConnell et al. 1982 (e14) | 1960–1981 | NA | Some | No | No | 20% |
| Aranha et al. 1983 (e3) | 1974–1981 | 97% | All | No | Yes | 29% |
| Agha 1984 (e8) | 1977–1982 | 80% | All | No | No | 20% |
| Warshaw und Rattner (4) | 1985 | NA | All | Yes | No | 21% |
| London et al. 1989 (e20) | 1984–1986 | NA | All | No | No | 64% |
| Bourliere und Sarles 1989 (e23) | 1972–1985 | 4% | All | Yes | Yes | 20% |
| Maringhini et al. 1999 (48) | 1986–1995 | 0% | All | No | Yes | 65% |
| Mehta et al. 2004 (e36) | 2001–2003 | 60% | Some | Yes | Yes | 60% |
| Cheruvu et al. 2003 (e33) | 1991–2002 | 14% | Some | Yes | Yes | 39% |
CT, computerized tomography; US, ultrasonography; ERCP, Endoscopic retrograde cholangiopancreatography; NA, data not available
Indications for treatment
When is there an indication for surgical, interventional, or endoscopic treatment? Treatment is indicated if one of the following complications is present:
compression of major vessels (with manifestations such as ischemic pain, a positive Hemoccult test due to ischemia, disturbed intestinal motility, a rise in the serum lactate concentration, or radiological demonstration of vascular compression),
compression of the stomach or duodenum leading to clinical symptoms,
stenosis of the common bile duct or impairment of biliary flow, with cholestasis,
infection of, or hemorrhage into, the cyst, or
a pancreaticopleural fistula.
Furthermore, treatment is definitely indicated for symptomatic cysts causing, for example, a feeling of abdominal distension, nausea and vomiting, pain, or gastrointestinal bleeding. A relative indication for treatment is present for pseudocysts greater than 4 cm in size with either unchanged size and morphology or progression over a period of more than 6 weeks (1). Pseudocysts of this size that have been present for this long rarely regress spontaneously and are increasingly likely to cause complications. The most suitable pseudocysts for endoscopic treatment are those with a wall thickness of less than 1 cm and more than 5 mm (14). In this range the pseudocyst wall can be readily penetrated with the endoscopic needle, yet are still stable enough for the insertion of pigtail stents.
A further relative indication for treatment is the presence of chronic pancreatitis with duct abnormalities or stones in the pancreatic duct. In these entities, constant irritation promotes inflammation and lowers the rate of spontaneous regression, which is no higher than 10% to 26% even for small cysts (11). Surgical treatment is urgently indicated whenever a malignant tumor is suspected (Boxes 1 and 2) (21).
Box 1. Indications for the Treatment of Pancreatic Pseudocysts.
Complicated pancreatic pseudocyst (one criterion suffices)
Compression of the abdominal great vessels (clinical manifestations or radiological evidence)
Clinically relevant gastric outlet stenosis or duodenal stenosis
Stenosis of the common bile duct with jaundice due to compression
Infected pancreatic pseudocyst (septic focus)
Hemorrhage into a pancreatic pseudocyst (danger of recurrent hemorrhage)
Pancreaticopleural fistula (risk of pneumonia, ARDS)
Symptomatic pancreatic pseudocyst
Abdominal distension
Nausea and vomiting
Pain
Upper gastrointestinal bleeding (10–20%)
Asymptomatic pancreatic pseudocyst
Pseudocyst >5cm, without any regression after more than 6 weeks of observation (1)
Cyst wall >5 mm (mature cyst) = high success rate of endoscopic or laparoscopic drainage (14)
Chronic pancreatitis with advanced pancreatic duct changes, pancreaticolithiasis = persistent irritation leading to inflammation, no more than 26% of cysts regress spontaneously in this situation; when they do not regress, the complication rate rises over the further course of illness (11)
Suspected cystic pancreatic tumor: median 5-year survival after early resection is good (63%) (21)
ARDS, acute respiratory distress syndrome
Box 2. Prerequisites and recommendations for the endoscopic drainage of pancreatic pseudocysts.
The distance between the pseudocyst and the gastric or duodenal wall should be less than 1 cm (15, 22).
The chosen approach should be through the site of greatest impression by the pseudocyst on the adjacent gastric or duodenal wall (22, e24).
Ideally, the cyst should be more than 5 cm in size and should cause impression of the gastric or duodenal wall; single cysts, mature cysts, and cysts without interruption of the pancreatic duct can be drained with high rates of success (24).
For the drainage of mature cysts, the pancreatic ductal system should first be investigated endoscopically, and transpapillary drainage is to be preferred whenever possible (24).
Symptomatic pancreatic pseudocysts: cysts that have been present for more than 6 weeks and have not regressed under conservative treatment should be treated (25).
Malignant lesions and pseudoaneurysms should always be ruled out before endoscopic treatment (15).
Treatment
The first successful operation for the drainage of a pancreatic pseudocyst was described by Bozeman in 1882. When these lesions are treated surgically, it usually suffices to incise and debride the cyst and to perform a partial resection so that tissue can be obtained for histopathology. The most important aspect of open surgical treatment is that the deepest point of the cyst should be drained or anastomosed whenever possible. This type of internal surgical cyst drainage, which may consist of a pseudocystoduodenostomy, a pseudocystogastrostomy, or a pseudocystojejunostomy, carries an average mortality of 2.5% and a morbidity of 16%. The rate of technical success is 90% to 100%, while the recurrence rate ranges from 0% to 12% over 96 months of postoperative follow-up and depends on the site of the pseudocyst as well as on the underlying illness (15).
A further development of the conventional surgical treatment of pancreatic pseudocysts makes use of a laparoscopic approach. 253 cases of laparoscopic pseudocyst drainage have been reported to date around the world. The reported rate of technical success is 92%, with 0% mortality, a 9% complication rate, and a 3% recurrence rate. In 6.7% of cases, the procedure had to be converted to an open laparotomy (16, e54) (table 2). A direct comparison of surgical and interventional techniques has so far been performed only for transcutaneous drainage, but not for endoscopic drainage (17). In general, surgical (including minimally invasive) techniques are hard to compare with transcutaneous interventional techniques, because of an evident selection bias. Patients that have been rejected as surgical candidates because of multimorbidity are often treated interventionally.
Table 2. The laparoscopic treatment of pancreatic pseudocysts.
| Reference | Number of patients | Success rate | Complete cyst drainage | Recurrence rate | Complications |
| Cuschieri et al. 1998 (e18) | 8 | 8 (100%) | 8 (100%) | 0 | 1 (13%) |
| Chowbey et al. 2001 (e38) | 5 | 5 (100%) | 5 (100%) | 0 | 0 |
| Ramachandran et al. 2002 (e28) | 5 | 5 (100%) | 5 (100%) | 0 | 0 |
| Park & Heniford et al. 2002 (e12) | 28 | 27 (96%) | 28 (100%) | 0 | 2 (7%) |
| Mori et al. 2002 (e17) | 17 | 14 (82%) | 17 (100%) | 1 (6%) | 3 (18%) |
| Fernandez-Cruz et al. 2002 (e30) | 6 | 6 (100%) | 6 (100%) | 0 | 0 |
| Zhou et al. 2003 (e7) | 13 | 12 (92%) | 13 (100%) | 1 (8%) | 0 |
| Teixeira et al. 2003 (e27) | 8 | 8 (100%) | 8 (100%) | 0 | 0 |
| Obermeyer et al. 2003 (e40) | 6 | 4 (67%) | 6 (100%) | 0 | 1 (17%) |
| Palanivelu et al. 2007 (37) | 108 | 98 (91%) | 107 (99%) | 1 (1%) | 9 (8%) |
| Hauters et al. 2004 (e11) | 17 | 16 (94%) | 15 (88%) | 0 | 2 (12%) |
| Davila-Cervantes et al. 2004 (e21) | 10 | 10 (100%) | 9 (90%) | 0 | 2 (20%) |
| Hindmarsh et al. 2005 (e16) | 15 | 12 (80%) | 14 (93%) | 2 (13%) | 2 (13%) |
| Owera & Ammori et al. 2008 (e1) | 7 | 7 (100%) | 7 (100%) | 0 | 0 |
| Total | 253 | 232 (92%) | 248 (98%) | 5 (3%) | 22 (9%) |
It has been repeatedly shown, however, that the complication rate in the treatment of chronic pancreatic pseudocysts is lower than that of acute pancreatic pseudocysts, and that this is true independently of the choice of therapeutic procedure.
Currently, transcutaneous drainage is indicated only as an emergency procedure for acute fluid retention or infected cysts, as the recurrence rate after this form of treatment ranges as high as 70% and percutaneous fistulae are a very common complication (more than 20% of cases).
The first alternative to surgery to be developed was percutaneous, endoscopically guided cyst drainage into the stomach (18). The endoscopic transpapillary approach to the pseudocyst is probably the least traumatic procedure. Thus, when a pancreatic pseudocyst is found to have a connection to Wirsung’s duct, the preferred treatment is often the transpapillary insertion of a stent for internal drainage.
There have not yet been any prospective, randomized trials on the question of the optimal timing of elective stent changes or the necessary duration of stent treatment for pancreatic pseudocysts (trials of this type have already been published for stents in the common bile duct). Depending on the individual report, 22% to 57% of pancreatic pseudocysts have been found to be connected to the pancreatic ductal system (13, e15). The current state of technology supports the performance of endoscopic retrograde pancreatography (ERP) to demonstrate a connection to the ductal system, or to rule out rupture of the pancreatic duct (as seen in 8% of cases after acute necrotizing pancreatitis), before endoscopic transmural drainage (19). Transmural drainage in the presence of an unrecognized rupture of the pancreatic duct, or when the pancreatic pseudocyst is connected to a stenotic pancreatic duct, is less likely to meet with long-term success.
The study of Arvanitakis made a major contribution to the treatment of pancreatic pseudocysts that have arisen through rupture of the pancreatic duct (19). He compared the effect of immediate stent removal with that of moderately prolonged stent drainage (the stents were left in place for a median duration of two months) after initially successful transgastric drainage. The result indicated that the stent should remain in place even after complete emptying of the pseudocyst, because the recurrence rate is significantly higher when it is removed early. This finding conflicts with the received opinion that stents should be removed as soon as possible after the emptying of a fluid collection because occlusion of the stent might lead to recurrence. In current practice, drainage stents in the pancreatic duct are changed every six weeks at the latest, and the treatment is continued for at least two months after regression of the pseudocyst.
The pre-interventional administration of antibiotics before endoscopic retrograde cholangiopancreatography (ERCP) is necessary whenever a pancreatic pseudocyst is suspected or is itself the indication for ERCP or ERP. If antibiotics are not given and the pseudocyst communicates with the pancreatic duct system, danger may arise from the retention of infected contrast medium. The study-specific incidence of infected pseudocysts and pancreatic abscesses rises if antibiotic prophylaxis is withheld (e15).
The endoscopic transgastric or transduodenal approach is a current alternative to a surgical procedure if transpapillary drainage of the pseudocyst is not possible. Since the first descriptions of this technique by Sahel 1987 and Cremer 1989, it has been tested in 1126 published cases and is now considered a safe and effective technique for the treatment of pancreatic pseudocysts, with a low complication rate in experienced hands. An important further development, which has also led to a broadening of the indications for this technique, is the use of endoscopic ultrasonography, including Doppler ultrasonography. When this is done, the rate of iatrogenic hemorrhage and perforation is lower, and the success rate is markedly higher. The reported success rate among 1126 patients was 79.2%, while the success rates in recent studies are well over 85%, which corresponds to the results of surgery. The mortality in larger series with more than 30 patients is 0.2%, while the recurrence rate is 7.6% and the complication rate is 12.8% (table 3).
Table 3. The endoscopic treatment of pancreatic pseudocysts.
| Reference | Number of patients | Success rate | Complete cyst drainage | Recurrence rate | Complications |
| Kozarek et al. 1985 (e9) | 4 | 2 (50%) | 2 (50%) | 0 | 1 (25%) died |
| Cremer et al. 1989 (e31) | 33 | 28 (85%) | 30 (91%) | 4 (12%) | 3 (9%) |
| Sahel et al. 1991 (e5) | 37 | 31 (86%) | 36 (97%) | 2 (5%) | 5 (14%) |
| Kozarek et al. 1991 (e45) | 14 | 11 (79%) | NA | 2 (14%) | 3 (21%) |
| Bejanin et al. 1993 (e46) | 26 | 19 (73%) | NA | 4 (15%) | 4 (15%) |
| Funnel et al. 1994 (e19) | 5 | 5 (100%) | 5 (100%) | 0 | 0 |
| Deviere et al. 1995 (e43) | 12 | 10 (87%) | 10 (87%) | 0 | 2 (13%) |
| Vitale et al. 1999 (e22) | 36 | 31 (86%) | 31 (86%) | 5 (14%) | 1 (3%) |
| White et al. 2000 (e47) | 20 | 20 (100%) | 20 (100%) | 0 | 2 (10%) |
| Giovannini et al. 2001 (e24) | 15 | 15 (100%) | 15 (100%) | 0 | 1 (6,6%) |
| Libera et al. 2000 (e6) | 25 | 21 (84%) | 20 (80%) | 1 (4%) | 6 (28%) |
| Norton et al. 2001 (e42) | 17 | 14 (82,4%) | 13 (76,5%) | 1 (7,1%) | 3 (17,6%) |
| Sharma et al. 2002 (e13) | 38 | 37 (97%) | 37 (97%) | 7 (16%) | 5 (13%) |
| Binmöller et al. 1995 (32) | 53 | 43 (81%) | 47 (89%) | 11 (23%) | 6 (11%) |
| Smits et al. 1995 (35) | 37 | 24 (65%) | 24 (65%) | 3 (12,5%) | 6 (16%) |
| Barthet et al. 1995 (e15) | 30 | 23 (77%) | 26 (87%) | 3 (11,5%) | 4 (13%) |
| Baron et al. 2002 (e44) | 64 | 52 (81%) | 59 (92%) | 7 (12%) | 11 (17%) |
| Catalano et al. 1995 (e25) | 21 | 16 (76%) | 17 (81%) | 1 (5%) | 1 (5%) |
| Antillon et al. 2006 (e29) | 33 | 31 (94%) | 24 (82%) | 1 (3%) | 2 (6%) |
| Hookey et al. 2006 (e41) | 116 | 102 (87,9%) | 108 (93,1%) | 19 (16,4%) | 13 (11%), |
| 6 (5,2%) died | |||||
| Krüger et al. 2006 (e34) | 35 | 33 (94%) | 30 (88%) | 4 (12%) | 0 |
| Weckmann et al. 2006 (e26) | 165 | 142 (86,1%) | 146 (86,1%) | 8 (5,3%) | 16 (10%) |
| Kahaleh et al. 2006 (e39) | 99 | 93 (94%) | NA | NA | 19 (19%) |
| Cahen et al. 2005 (e49) | 92 | 89 (97%) | 79 (86%) | 4 (5%) | 31 (35%) |
| 1 (1%) died | |||||
| Total | 1126 | 892 (79,2%) | 779 (87,3%) | 86 (7,6%) | 144 (12,8%) |
NA, data not available
The further technical development of endoscopic techniques is still in progress. In a recently published prospective study involving 116 patients, Deviere and colleagues (e41) drew the following conclusions:
The treatment of pancreatic pseudocysts should always involve an interdisciplinary therapeutic approach.
Endoscopic interventions are more often successful when multiple wide stents are placed, and this does not elevate either morbidity or mortality. This is particularly true with regard to the placement of pigtail catheters
Pigtail catheters are preferable to straight stents, because their complication rate is markedly lower (e55).
Which procedure, when?
When fluid retention occurs, as may happen in the early phase of acute pancreatitis, transcutaneous or endoscopic drainage is indicated only in rare, exceptional cases. The success rates of transcutaneous drainage range from 42% to 96%. The morbidity and mortality are low, but the recurrence rate is high (24%), and the likelihood of a persistent pancreaticocutaneous fistula should not be underestimated. Only relatively few data on endoscopic drainage for this indication have been published to date. The procedure seems promising, however, and would be an appropriate way of lowering the rate of secondary infectious complications. The transgastric endoscopic approach to infected pancreatic necroses and fluid collections in acute pancreatitis in now increasingly the procedure of choice, including in the authors’ institution.
In cases of symptomatic or complicated acute pancreatic pseudocyst, or of an acute pseudocyst measuring greater than 5 cm that has been present for more than 6 weeks, an endoscopic procedure should be chosen preferably via a transpapillary or transmural approach, although drainage through the gastric wall has the highest rate of technical success. Operative pseudocyst drainage, because of its higher mortality, should be used as the primary treatment only in rare cases or when the pseudocyst extends far down into the pelvis.
The treatment criteria listed in Table 3 are also valid for the treatment of symptomatic or complicated chronic pancreatic pseudocysts or of chronic pseudocysts greater than 5 cm in size (figure 2). It must be borne in mind, however, that chronic pseudocysts regress spontaneously much less commonly than acute ones. Moreover, the surgical treatment of pseudocysts due to chronic pancreatitis carries a markedly lower mortality and morbidity. In this situation, laparoscopic pseudocystojejunostomy is gradually becoming established as an alternative to open surgery. In highly experienced laparoscopic centers, endoscopic drainage yields comparable results with respect to effectiveness and safety. There have not yet been any published reports from Germany concerning the results of laparoscopic pseudocyst drainage.
Figure 2.
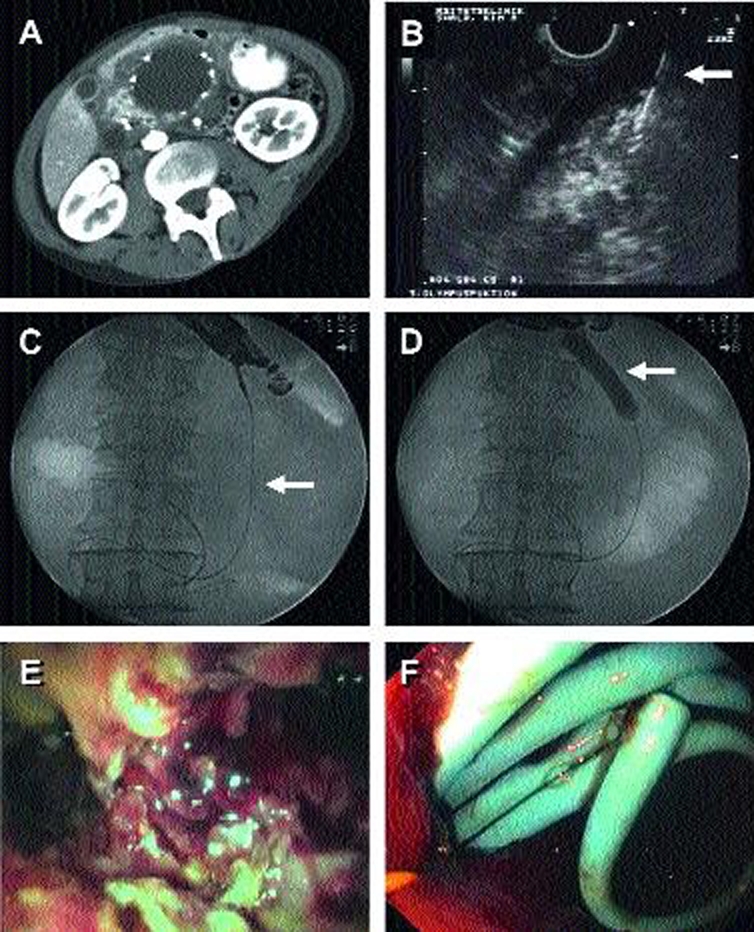
The individual steps in the endoscopic treatment of a large pancreatic pseudocyst are shown.
(a) A CT scan shows the head of the pancreas with a symptomatic pancreatic pseudocyst due to chronic pancreatitis. There are calcifications in the pseudocyst wall.
(b) An avascular area of the gastric wall is located endosonographically and the pseudocyst is punctured with a needle (arrow).
(c) A wire (arrow) is introduced through the needle into the pseudocyst; a dilating balloon (arrow in [d]) is then introduced over the wire so that the path of approach can be widened to the diameter of the endoscope.
(d) Introduction of the dilatation balloon (arrow)
(e) View through the endoscope into the pancreatic pseudocyst, with the opportunity to take biopsies to rule out malignancy
(f) The endoscopic path of approach is held open by the introduction of pigtail catheters (usually two to four) between the stomach and the pancreatic pseudocyst; this prevents rapid spontaneous closure and enables regression of the pseudocyst.
Pancreatic abscesses (collections of pus in the retroperitoneal space) can be drained either transcutaneously or transmurally. Large case series are available only for transcutaneous drainage (success rate up to 87%, complication rate 4% to 17%, mortality 8%). Operative treatment carries a higher mortality. Here, too, the authors currently prefer to use the transgastric endoscopic approach in their own institution.
Pancreatic pseudocyst versus cystic malignant tumor
The differential diagnosis of a pancreatic pseudocyst from a cystic malignancy is difficult. In addition to obtaining cyst fluid by puncture, there are a number of further criteria that can aid in the differential diagnosis: prior episodes of acute pancreatitis, or known chronic pancreatitis, makes a pancreatic pseudocyst more likely; cystic malignant tumors are more common in women than in men. Weight loss, a palpable mass, and the lack of pre-existing pancreatic disease all make a malignant tumor more likely. Pancreatic pseudocysts more often have a calcified cyst wall, usually lie in the head of the pancreas, and usually have a wall that is less than 1 cm thick. Cystic malignant tumors, on the other hand, are often multilocular and tend to arise in the body and tail of the pancreas; their walls are less commonly calcified and more often over 1 cm thick, with nodular components. An important criterion for malignancy is a markedly elevated CEA value in the cyst fluid (e56). In general, the rule applies: When in doubt about the possibility of malignancy, operate.
Guidelines
There are no current guidelines for the treatment of pancreatic pseudocysts in either the German-speaking or the English-speaking countries. An update of the older guidelines of the German Society for Digestive and Metabolic Diseases (Deutsche Gesellschaft für Verdauungs- und Stoffwechselkrankheiten, DGVS) is planned to appear in 2009. Even today, most of the data are derived from uncontrolled, mostly retrospective case series (evidence level III). Randomized, prospective trials (19, 20) are urgently needed.
Overview
The endoscopic and minimally invasive therapeutic procedures for the drainage of pancreatic pseudocysts are superior to open surgical techniques with respect to their success rates, morbidity, and mortality, but they can not always be performed (box 2). In making treatment decisions, it is important to recall that 50% of pancreatic pseudocysts do not require any intervention and can be successfully managed by a wait-and-watch approach (table 1). Laparoscopic and endoscopic drainage have comparable success rates, while that of transcutaneous drainage is somewhat worse. Thus, the choice of technique depends very heavily on the experience of the treatment center. In all cases where a malignant tumor is suspected, open surgery should be performed. Pancreatic pseudocysts require treatment when they cause symptoms, produce complications, or have reached a size exceeding 5 cm and do not regress after 6 weeks of observation (box 1). In the last-named situation, treatment is indicated because complications can otherwise be expected.
Key messages.
About 50% of pancreatic pseudocysts regress spontaneously and need no treatment.
If treatment is indicated (box 1), endoscopic and laparoscopic therapeutic procedures have comparable results, while open surgery carries a somewhat higher morbidity and mortality.
Endoscopic pseudocyst drainage with the aid of pigtail catheters by way of the pancreatic duct or the gastric wall (less commonly, the duodenal wall) is currently the safest and most frequently used technique.
There are no randomized studies directly comparing the therapeutic techniques.
Whenever a cystic malignant tumor is suspected, open surgery should be chosen.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of Interest Statement
The authors declare that they have no conflict of interest as defined by the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Bradley EL., 3rd A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 2.Imrie CW, Buist LJ, Shearer MG. Importance of cause in the outcome of pancreatic pseudocysts. Am J Surg. 1988;156:159–162. doi: 10.1016/s0002-9610(88)80055-2. [DOI] [PubMed] [Google Scholar]
- 3.Maringhini A, Uomo G, Patti R, et al. Pseudocysts in acute nonalcoholic pancreatitis: incidence and natural history. Dig Dis Sci. 1999;44:1669–1673. doi: 10.1023/a:1026691700511. [DOI] [PubMed] [Google Scholar]
- 4.Barthet M, Bugallo M, Moreira LS, Bastid C, Sastre B, Sahel J. Management of cysts and pseudocysts complicating chronic pancreatitis. A retrospective study of 143 patients. Gastroenterol Clin Biol. 1993;17:270–276. [PubMed] [Google Scholar]
- 5.Ammann RW, Akovbiantz A, Largiader F, Schueler G. Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology. 1984;86:820–828. [PubMed] [Google Scholar]
- 6.Andren-Sandberg A, Dervenis C. Surgical treatment of pancreatic pseudocysts in the 2000’s-laparoscopic approach. Acta Chir Iugosl. 2003;50:21–26. doi: 10.2298/aci0304021a. [DOI] [PubMed] [Google Scholar]
- 7.O’Malley VP, Cannon JP, Postier RG. Pancreatic pseudocysts: cause, therapy, and results. Am J Surg. 1985;150:680–682. doi: 10.1016/0002-9610(85)90407-6. [DOI] [PubMed] [Google Scholar]
- 8.Balthazar EJ, Freeny PC, vanSonnenberg E. Imaging and intervention in acute pancreatitis. Radiology. 1994;193:297–306. doi: 10.1148/radiology.193.2.7972730. [DOI] [PubMed] [Google Scholar]
- 9.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Bradley EL, Gonzalez AC, Clements JL., Jr Acute pancreatic pseudocysts: incidence and implications. Ann Surg. 1976;184:734–737. doi: 10.1097/00000658-197612000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gouyon B, Levy P, Ruszniewski P, et al. Predictive factors in the outcome of pseudocysts complicating alcoholic chronic pancreatitis. Gut. 1997;41:821–825. doi: 10.1136/gut.41.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warshaw AL, Rattner DW. Timing of surgical drainage for pancreatic pseudocyst. Clinical and chemical criteria. Ann Surg. 1985;202:720–724. doi: 10.1097/00000658-198512000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nealon WH, Walser E. Main pancreatic ductal anatomy can direct choice of modality for treating pancreatic pseudocysts (surgery versus percutaneous drainage) Ann Surg. 2002;235:751–758. doi: 10.1097/00000658-200206000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beckingham IJ, Krige JE, Bornman PC, Terblanche J. Long term out-come of endoscopic drainage of pancreatic pseudocysts. Am J Gastroenterol. 1999;94:71–74. doi: 10.1111/j.1572-0241.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- 15.Rosso E, Alexakis N, Ghaneh P, et al. Pancreatic pseudocyst in chronic pancreatitis: endoscopic and surgical treatment. Dig Surg. 2003;20:397–406. doi: 10.1159/000072706. [DOI] [PubMed] [Google Scholar]
- 16.Palanivelu C, Senthilkumar K, Madhankumar MV, et al. Management of pancreatic pseudocyst in the era of laparoscopic surgery—Experience from a tertiary centre. Surg Endosc. 2007;21:2262–2267. doi: 10.1007/s00464-007-9365-y. [DOI] [PubMed] [Google Scholar]
- 17.Morton JM, Brown A, Galanko JA, Norton JA, Grimm IS, Behrns KE. A national comparison of surgical versus percutaneous drainage of pancreatic pseudocysts: 1997-2001. J Gastrointest Surg. 2005;9:15–20. doi: 10.1016/j.gassur.2004.10.005. discussion 20-1. [DOI] [PubMed] [Google Scholar]
- 18.Kozarek RA. Endoscopically placed pancreaticobiliary conduits: diagnostic and therapeutic uses. J Clin Gastroenterol. 1982;4:497–501. doi: 10.1097/00004836-198212000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Arvanitakis M, Delhaye M, Bali MA, Matos C, De Maertelaer V, Le Moine O, Deviere J. Pancreatic-fluid collections: a randomized controlled trial regarding stent removal after endoscopic transmural drainage. Gastrointest Endosc. 2007;65:609–619. doi: 10.1016/j.gie.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 20.Cahen D, Rauws E, Fockens P, Weverling G, Huibregtse K, Bruno M. Endoscopic drainage of pancreatic pseudocysts: long-term outcome and procedural factors associated with safe and successful treatment. Endoscopy. 2005;37:977–983. doi: 10.1055/s-2005-870336. [DOI] [PubMed] [Google Scholar]
- 21.Levy P, Jouannaud V, O’Toole D, et al. Natural history of intraductal papillary mucinous tumors of the pancreas: actuarial risk of malignancy. Clin Gastroenterol Hepatol. 2006;4:460–468. doi: 10.1016/j.cgh.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Smits ME, Rauws EA, Tytgat GN, Huibregtse K. The efficacy of endo-scopic treatment of pancreatic pseudocysts. Gastrointest Endosc. 1995;42:202–207. doi: 10.1016/s0016-5107(95)70092-7. [DOI] [PubMed] [Google Scholar]
- 23.Monkemuller KE, Baron TH, Morgan DE. Transmural drainage of pancreatic fluid collections without electrocautery using the Seldinger technique. Gastrointest Endosc. 1998;48:195–200. doi: 10.1016/s0016-5107(98)70164-6. [DOI] [PubMed] [Google Scholar]
- 24.Chak A. Endosonographic-guided therapy of pancreatic pseudocysts. Gastrointest Endosc. 2000;52:23–27. doi: 10.1067/mge.2000.110719. [DOI] [PubMed] [Google Scholar]
- 25.Binmoeller KF, Seifert H, Walter A, Soehendra N. Transpapillary and transmural drainage of pancreatic pseudocysts. Gastrointest Endosc. 1995;42:219–224. doi: 10.1016/s0016-5107(95)70095-1. [DOI] [PubMed] [Google Scholar]
- e1.Owera AM, Ammori BJ. Laparoscopic endogastric and transgastric cystgastrostomy and pancreatic necrosectomy. Hepatogastroenterology. 2008;55:262–265. [PubMed] [Google Scholar]
- e2.Pollak EW, Michas CA, Wolfman EF., Jr Pancreatic pseudocyst: management in fifty-four patients. Am J Surg. 1978;135:199–201. doi: 10.1016/0002-9610(78)90097-1. [DOI] [PubMed] [Google Scholar]
- e3.Aranha GV, Prinz RA, Esguerra AC, Greenlee HB. The nature and course of cystic pancreatic lesions diagnosed by ultrasound. Arch Surg. 1983;118:486–488. doi: 10.1001/archsurg.1983.01390040090019. [DOI] [PubMed] [Google Scholar]
- e4.Warshaw AL, Rattner DW. Timing of surgical drainage for pancreatic pseudocyst. Clinical and chemical criteria. Ann Surg. 1985;202:720–724. doi: 10.1097/00000658-198512000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e5.Sahel J. Endoscopic drainage of pancreatic cysts. Endoscopy. 1991;23:181–184. doi: 10.1055/s-2007-1010651. [DOI] [PubMed] [Google Scholar]
- e6.Libera ED, Siqueira ES, Morais M, Rohr MR, Brant CQ, Ardengh JC, Ferrari AP. Pancreatic pseudocysts transpapillary and transmural drainage. HPB Surg. 2000;11:333–338. doi: 10.1155/2000/43853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e7.Zhou ZG, Zheng YC, Shu Y, Hu WM, Tian BL, Li QS, Zhang ZD. Laparoscopic management of severe acute pancreatitis. Pancreas. 2003;27:e46–e50. doi: 10.1097/00006676-200310000-00019. [DOI] [PubMed] [Google Scholar]
- e8.Agha FP. Spontaneous resolution of acute pancreatic pseudocysts. Surg Gynecol Obstet. 1984;158:22–26. [PubMed] [Google Scholar]
- e9.Kozarek RA, Brayko CM, Harlan J, Sanowski RA, Cintora I, Kovac A. Endoscopic drainage of pancreatic pseudocysts. Gastrointest Endosc. 1985;31:322–327. doi: 10.1016/s0016-5107(85)72215-8. [DOI] [PubMed] [Google Scholar]
- e10.Czaja AJ, Fisher M, Marin GA. Spontaneous resolution of pancreatic masses (pseudocysts?)—development and disappearance after acute alcoholic pancreatitis. Arch Intern Med. 1975;135:558–562. [PubMed] [Google Scholar]
- e11.Hauters P, Weerts J, Navez B, Champault G, Peillon C, Totte E, et al. Laparoscopic treatment of pancreatic pseudocysts. Surg Endosc. 2004;18:1645–1648. doi: 10.1007/s00464-003-9280-9. [DOI] [PubMed] [Google Scholar]
- e12.Park AE, Heniford BT. Therapeutic laparoscopy of the pancreas. Ann Surg. 2002;236:149–158. doi: 10.1097/00000658-200208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e13.Sharma SS, Bhargawa N, Govil A. Endoscopic management of pancreatic pseudocyst: a longterm follow-up. Endoscopy. 2002;34:203–207. doi: 10.1055/s-2002-20292. [DOI] [PubMed] [Google Scholar]
- e14.McConnell DB, Gregory JR, Sasaki TM, Vetto RM. Pancreatic pseudocyst. Am J Surg. 1982;143:599–601. doi: 10.1016/0002-9610(82)90172-6. [DOI] [PubMed] [Google Scholar]
- e15.Barthet M, Sahel J, Bodiou-Bertei C, Bernard JP. Endoscopic transpapillary drainage of pancreatic pseudocysts. Gastrointest Endosc. 1995;42:208–213. doi: 10.1016/s0016-5107(95)70093-5. [DOI] [PubMed] [Google Scholar]
- e16.Hindmarsh A, Lewis MP, Rhodes M. Stapled laparoscopic cyst-gastrostomy: a series with 15 cases. Surg Endosc. 2005;9:143–147. doi: 10.1007/s00464-004-9042-3. [DOI] [PubMed] [Google Scholar]
- e17.Mori T, Abe N, Sugiyama M, Atomi Y. Laparoscopic pancreatic cystgastrostomy. J Hepatobiliary Pancreat Surg. 2002;9:548–554. doi: 10.1007/s005340200072. [DOI] [PubMed] [Google Scholar]
- e18.Cuschieri SA, Jakimowicz JJ, Stultiens G. Laparoscopic infracolic approach for complications of acute pancreatitis. Semin Laparosc Surg. 1998;5:189–194. doi: 10.1177/155335069800500306. [DOI] [PubMed] [Google Scholar]
- e19.Funnell IC, Bornman PC, Krige JE, Beningfield SJ, Terblanche J. Endoscopic drainage of traumatic pancreatic pseudocyst. Br J Surg. 1994;81:879–881. doi: 10.1002/bjs.1800810628. [DOI] [PubMed] [Google Scholar]
- e20.London NJ, Neoptolemos JP, Lavelle J, Bailey I, James D. Serial computed tomography scanning in acute pancreatitis: a prospective study. Gut. 1989;30:397–403. doi: 10.1136/gut.30.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e21.Davila-Cervantes A, Gomez F, Chan C, Bezaury P, Robles-Diaz G, Uscanga LF, Herrera MF. Laparoscopic drainage of pancreatic pseudocysts. Surg Endosc. 2004;18:1420–1426. doi: 10.1007/s00464-003-8204-z. [DOI] [PubMed] [Google Scholar]
- e22.Vitale GC, Lawhon JC, Larson GM, Harrell DJ, Reed DN, Jr., MacLeod S. Endoscopic drainage of the pancreatic pseudocyst. Surgery. 1999;126:616–621. discussion 621-3. [PubMed] [Google Scholar]
- e23.Bourliere M, Sarles H. Pancreatic cysts and pseudocysts associated with acute and chronic pancreatitis. Dig Dis Sci. 1989;34:343–348. doi: 10.1007/BF01536253. [DOI] [PubMed] [Google Scholar]
- e24.Giovannini M, Pesenti C, Rolland AL, Moutardier V, Delpero JR. Endoscopic ultrasoundguided drainage of pancreatic pseudocysts or pancreatic abscesses using a therapeutic echo endoscope. Endoscopy. 2001;33:473–477. doi: 10.1055/s-2001-14967. [DOI] [PubMed] [Google Scholar]
- e25.Catalano MF, Geenen JE, Schmalz MJ, Johnson GK, Dean RS, Hogan WJ. Treatment of pancreatic pseudocysts with ductal communication by transpapillary pancreatic duct endoprosthesis. Gastrointest Endosc. 1995;42:214–218. doi: 10.1016/s0016-5107(95)70094-3. [DOI] [PubMed] [Google Scholar]
- e26.Weckman L, Kylanpaa ML, Puolakkainen P, Halttunen J. Endoscopic treatment of pancreatic pseudocysts. Surg Endosc. 2006;20:603–607. doi: 10.1007/s00464-005-0201-y. [DOI] [PubMed] [Google Scholar]
- e27.Teixeira J, Gibbs KE, Vaimakis S, Rezayat C. Laparoscopic Roux-en-Y pancreatic cystjejunostomy. Surg Endosc. 2003;17:1910–1913. doi: 10.1007/s00464-003-8801-x. [DOI] [PubMed] [Google Scholar]
- e28.Ramachandran CS, Goel D, Arora V, Kumar M. Gastroscopic-assisted laparoscopic cystogastrostomy in the management of pseudocysts of the pancreas. Surg Laparosc Endosc Percutan Tech. 2002;12:433–436. doi: 10.1097/00129689-200212000-00009. [DOI] [PubMed] [Google Scholar]
- e29.Antillon MR, Shah RJ, Stiegmann G, Chen YK. Single-step EUS-guided transmural drainage of simple and complicated pancreatic pseudocysts. Gastrointest Endosc. 2006;63:797–803. doi: 10.1016/j.gie.2005.10.025. [DOI] [PubMed] [Google Scholar]
- e30.Fernandez-Cruz L, Saenz A, Astudillo E, Pantoja JP, Uzcategui E, Navarro S. Laparoscopic pancreatic surgery in patients with chronic pancreatitis. Surg Endosc. 2002;16:996–1003. doi: 10.1007/s00464-001-9065-y. [DOI] [PubMed] [Google Scholar]
- e31.Cremer M, Deviere J, Engelholm L. Endoscopic management of cysts and pseudocysts in chronic pancreatitis: long-term follow-up after 7 years of experience. Gastrointest Endosc. 1989;35:1–9. doi: 10.1016/s0016-5107(89)72677-8. [DOI] [PubMed] [Google Scholar]
- e32.Binmoeller KF, Seifert H, Walter A, Soehendra N. Transpapillary and transmural drainage of pancreatic pseudocysts. Gastrointest Endosc. 1995;42:219–224. doi: 10.1016/s0016-5107(95)70095-1. [DOI] [PubMed] [Google Scholar]
- e33.Cheruvu CV, Clarke MG, Prentice M, Eyre-Brook IA. Conservative treatment as an option in the management of pancreatic pseudocyst. Ann R Coll Surg Engl. 2003;85:313–316. doi: 10.1308/003588403769162413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e34.Kruger M, Schneider AS, Manns MP, Meier PN. Endoscopic management of pancreatic pseudocysts or abscesses after an EUS-guided 1-step procedure for initial access. Gastrointest Endosc. 2006;63:409–416. doi: 10.1016/j.gie.2005.11.047. [DOI] [PubMed] [Google Scholar]
- e35.Smits ME, Rauws EA, Tytgat GN, Huibregtse K. The efficacy of endoscopic treatment of pancreatic pseudocysts. Gastrointest Endosc. 1995;42:202–207. doi: 10.1016/s0016-5107(95)70092-7. [DOI] [PubMed] [Google Scholar]
- e36.Mehta R, Suvarna D, Sadasivan S, John A, Raj V, Nair P, Balakrishnan V. Natural course of asymptomatic pancreatic pseudocyst: a prospective study. Indian J Gastroenterol. 2004;23:140–142. [PubMed] [Google Scholar]
- e37.Palanivelu C, Senthilkumar K, Madhankumar MV, Rajan PS, Shetty AR, Jani K, Rangarajan M, Maheshkumaar GS. Management of pancreatic pseudocyst in the era of laparoscopic surgery—experience from a tertiary centre. Surg Endosc. 2007;21:2262–2267. doi: 10.1007/s00464-007-9365-y. [DOI] [PubMed] [Google Scholar]
- e38.Chowbey PK, Soni V, Sharma A, Khullar R, Baijal M, Vashistha A. Laparoscopic intragastric stapled cystogastrostomy for pancreatic pseudocyst. J Laparoendosc Adv Surg Tech A. 2001;11:201–205. doi: 10.1089/109264201750539709. [DOI] [PubMed] [Google Scholar]
- e39.Kahaleh M, Shami VM, Conaway MR, Tokar J, Rockoff T, De La Rue SA, de Lange E, Bassignani M, Gay S, Adams RB, Yeaton P. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38:355–359. doi: 10.1055/s-2006-925249. [DOI] [PubMed] [Google Scholar]
- e40.Obermeyer RJ, Fisher WE, Salameh JR, Jeyapalan M, Sweeney JF, Brunicardi FC. Laparoscopic pancreatic cystogastrostomy. Surg Laparosc Endosc Percutan Tech. 2003;13:250–253. doi: 10.1097/00129689-200308000-00005. [DOI] [PubMed] [Google Scholar]
- e41.Hookey LC, Debroux S, Delhaye M, Arvanitakis M, Le Moine O, Deviere J. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006;63:635–643. doi: 10.1016/j.gie.2005.06.028. [DOI] [PubMed] [Google Scholar]
- e42.Norton ID, Clain JE, Wiersema MJ, DiMagno EP, Petersen BT, Gostout CJ. Utility of endoscopic ultrasonography in endoscopic drainage of pancreatic pseudocysts in selected patients. Mayo Clin Proc. 2001;76:794–798. doi: 10.1016/S0025-6196(11)63223-0. [DOI] [PubMed] [Google Scholar]
- e43.Deviere J, Bueso H, Baize M, Azar C, Love J, Moreno E, Cremer M. Complete disruption of the main pancreatic duct: endoscopic management. Gastrointest Endosc. 1995;42:445–451. doi: 10.1016/s0016-5107(95)70048-x. [DOI] [PubMed] [Google Scholar]
- e44.Baron TH, Harewood GC, Morgan DE, Yates MR. Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc. 2002;56:7–17. doi: 10.1067/mge.2002.125106. [DOI] [PubMed] [Google Scholar]
- e45.Kozarek RA, Ball TJ, Patterson DJ, Freeny PC, Ryan JA, Traverso LW. Endoscopic transpapillary therapy for disrupted pancreatic duct and peripancreatic fluid collections. Gastroenterology. 1991;100:1362–1370. [PubMed] [Google Scholar]
- e46.Bejanin H, Liguory C, Ink O, Fritsch J, Choury AD, Lefebvre JF, Vilgrain V, Etienne JP. Endoscopic drainage of pseudocysts of the pancreas. Study of 26 cases. Gastroenterol Clin Biol. 1993;17:804–810. [PubMed] [Google Scholar]
- e47.White SA, Sutton CD, Berry DP, Chillistone D, Rees Y, Dennison AR. Experience of combined endoscopic percutaneous stenting with ultrasound guidance for drainage of pancreatic pseudocycts. Ann R Coll Surg Engl. 2000;82:11–15. [PMC free article] [PubMed] [Google Scholar]
- e48.Maringhini A, Uomo G, Patti R, Rabitti P, Termini A, Cavallera A, Dardanoni G, Manes G, Ciambra M, Laccetti M, Biffarella P, Pagliaro L. Pseudocysts in acute nonalcoholic pancreatitis: incidence and natural history. Dig Dis Sci. 1999;44:1669–1673. doi: 10.1023/a:1026691700511. [DOI] [PubMed] [Google Scholar]
- e49.Cahen D, Rauws E, Fockens P, Weverling G, Huibregtse K, Bruno M. Endoscopic drainage of pancreatic pseudocysts: long-term outcome and procedural factors associated with safe and successful treatment. Endoscopy. 2005;37:977–983. doi: 10.1055/s-2005-870336. [DOI] [PubMed] [Google Scholar]
- e50.Lee JK, Stanley RJ, Melson GL, Sagel SS. Pancreatic imaging by ultrasound and computed tomography: a general review. Radiol Clin North Am. 1979;17:105–117. [PubMed] [Google Scholar]
- e51.Goulet RJ, Goodman J, Schaffer R, Dallemand S, Andersen DK. Multiple pancreatic pseudocyst disease. Ann Surg. 1984;199:6–13. doi: 10.1097/00000658-198401000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e52.Lehman GA. Pseudocysts. Gastrointest Endosc. 1999;49:81–84. doi: 10.1016/s0016-5107(99)70533-x. [DOI] [PubMed] [Google Scholar]
- e53.Linder JD, Geenen JE, Catalano MF. Cyst fluid analysis obtained by EUS-guided FNA in the evaluation of discrete cystic neoplasms of the pancreas: a prospective single-center experience. Gastrointest Endosc. 2006;64:697–702. doi: 10.1016/j.gie.2006.01.070. [DOI] [PubMed] [Google Scholar]
- e54.Bhattacharya D, Ammori BJ. Minimally invasive approaches to the management of pancreatic pseudocysts: review of the literature. Surg Laparosc Endosc Percutan Tech. 2003;13:141–148. doi: 10.1097/00129689-200306000-00001. [DOI] [PubMed] [Google Scholar]
- e55.Giovannini M. What is the best endoscopic treatment for pancreatic pseudocysts? Gastrointest Endosc. 2007;65:620–623. doi: 10.1016/j.gie.2006.12.046. [DOI] [PubMed] [Google Scholar]
- e56.Brugge WR. Optimizing methods for the diagnosis of pancreatic cancer. J Clin Gastroenterol. 2007;41:869–870. doi: 10.1097/MCG.0b013e318146849f. [DOI] [PubMed] [Google Scholar]