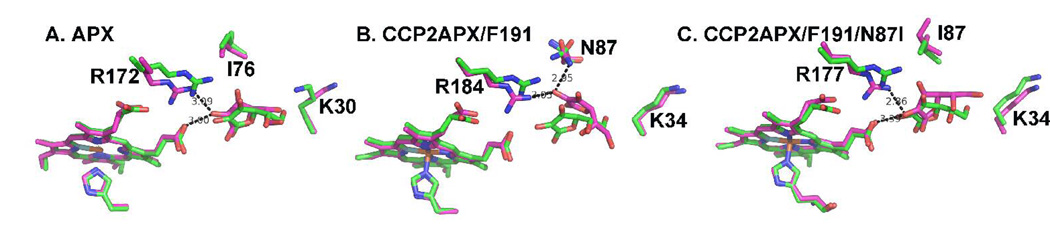
Fig. 3.
Average MD structures (magenta) superimposed on original structures (green) for (A) APX, (B) CCP2APX and (C) CCP2APX with Asn 87 converted to Ile. Note that in APX (panel A) the ascorbate remains stable throughout the MD simulations while in CCP2APX (panel B) the ascorbate moves up toward Asn 87 thus allowing the ascorbate and Asn80 to form an H-bond. The movement of the substrate results in the loss of the heme-ascorbate H-bond. The in silico conversion of Asn 87 to Ile in CCP2APX results in the ascorbate maintaining its interactions with Arg 184 and the heme.