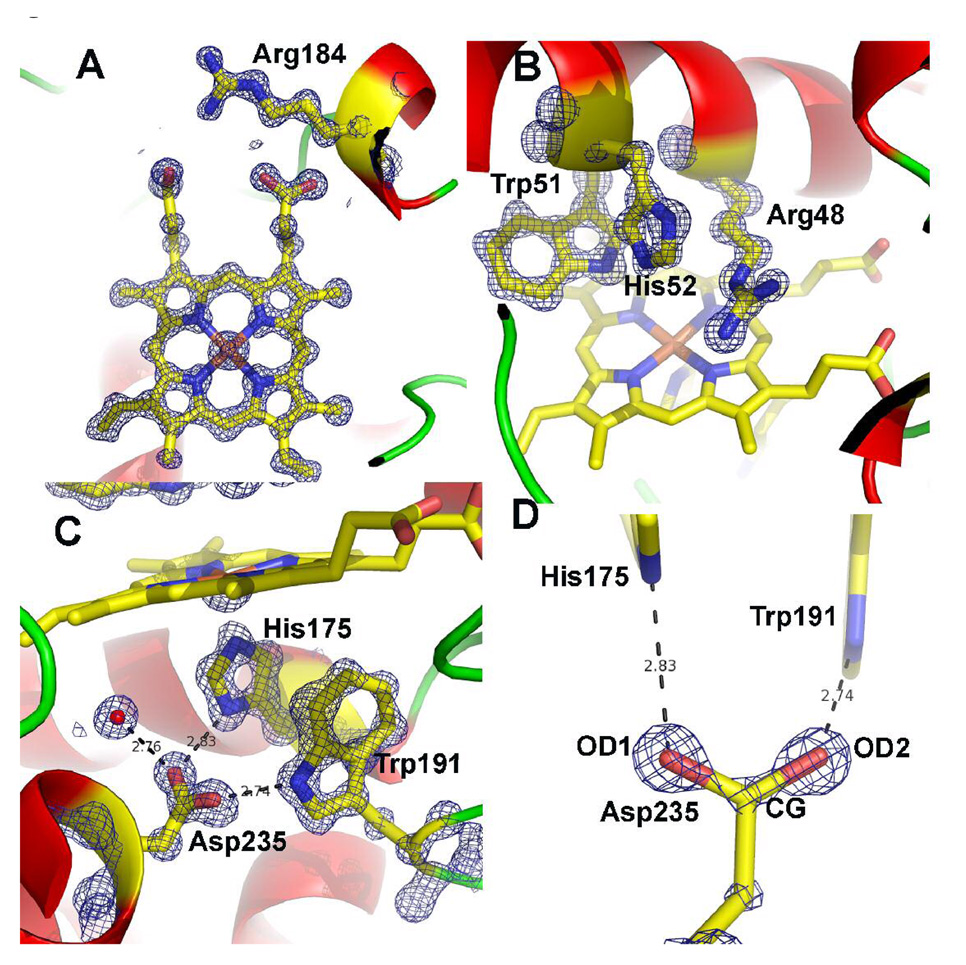
Fig. 4.
1.06 Å 2Fo−Fc maps for the CCP/R184 mutant contoured at 2.5 σ in panels A, B, and C and 5.0 σ in panel D. A) The region around the mutant side chain Arg 184. B) The distal pocket showing the conserved residues Arg 48 and His 52 that are the catalytic groups responsible for heterolytic cleavage of the peroxide O–O bond and formation of compound I. C) The proximal binding pocket showing the conserved His175 ligand and its H-bonding partner Asp235. Trp 191 is the site of free radical formation in compound I. D). The 2Fo−Fc map contoured at 5.0 σ after 10 rounds of refinement with no angle or distance restraints applied to Asp 235. Note the continuous density along the CG-OD2 bond suggesting a double bond while the weaker connectivity between CG and OD1 indicates a single bond.