Abstract
The stereospecific intramolecular alkylation of a hydroperoxyacetal provides the basis for the first asymmetric synthesis of the dioxane propionate core of the peroxyplakorates. Chemoselective hydrometallation of an alkyne in the presence of a peroxide is used to introduce a synthon for the polyunsaturated side chains of the peroxyplakorates. The route suggests a general solution for the 1,2-dioxane unit in many peroxide natural products.
Keywords: Peroxyplakoric; 1,2-dioxane; peroxide; intramolecular alkylation
1.0 Introduction
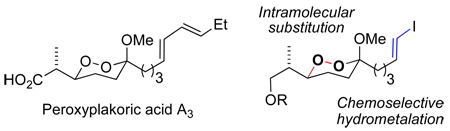
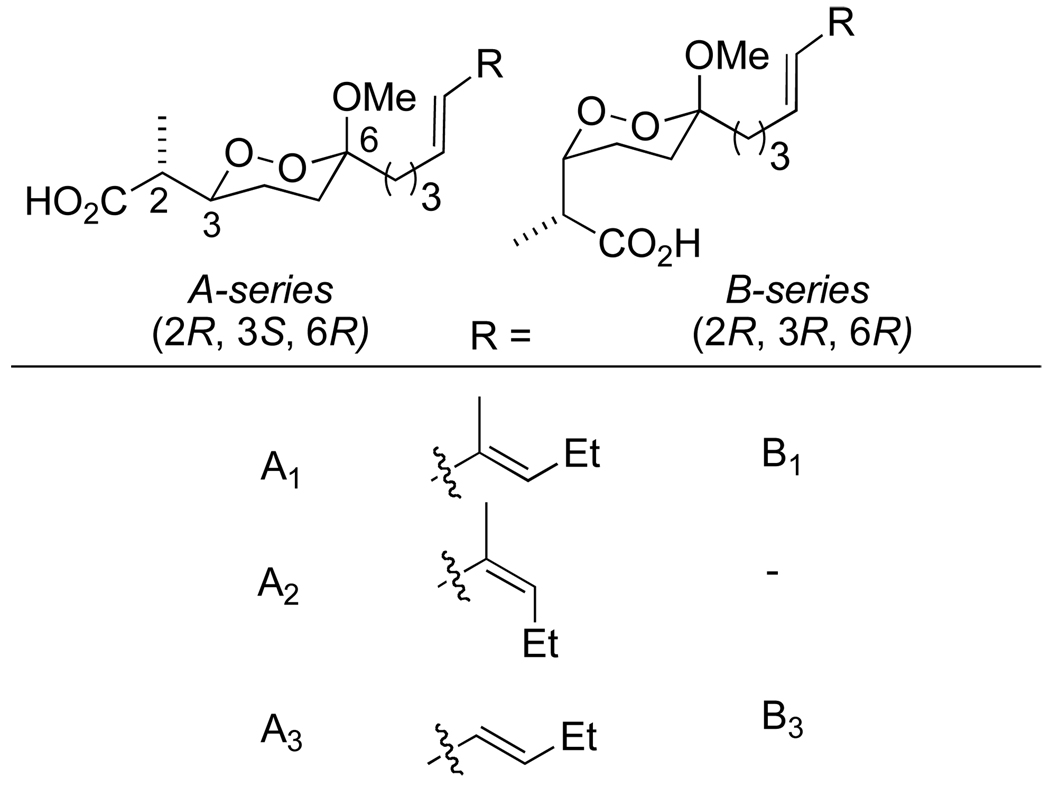
The peroxyplakoric acids are a family of marine natural products sharing a 6-methoxy-1,2-dioxane-3-propionic acid core, 1 , 2 and differing in the stereochemistry at C3 and in the geometry and substitution of the diene within the C6 tail (Figure 1). Several members of the family, as well as some simplified analogs, possess significant antifungal or antimalarial activity.3,4 However, there has been no reported synthesis of a peroxyplakoric acid,5 nor has there been a general strategy for asymmetric synthesis of alkoxy-1,2-dioxanes.6 We now report an asymmetric synthesis of 6-alkoxy-3,6-dialkyl-1,2-dioxanes by a route that should be broadly applicable to 1,2-dioxanes, one of the most common substructures in peroxide natural products.1,7,8
Figure 1.
Peroxyplakoric acids
2.0 Background
The two major challenges in the asymmetric synthesis of the peroxyplakorates are stereoselective introduction of the 3-alkoxy-3,6-dialkyl substituted 1,2-dioxane, and incorporation of both an oxidation-sensitive side chain and a reduction-sensitive 1,2-dioxane.
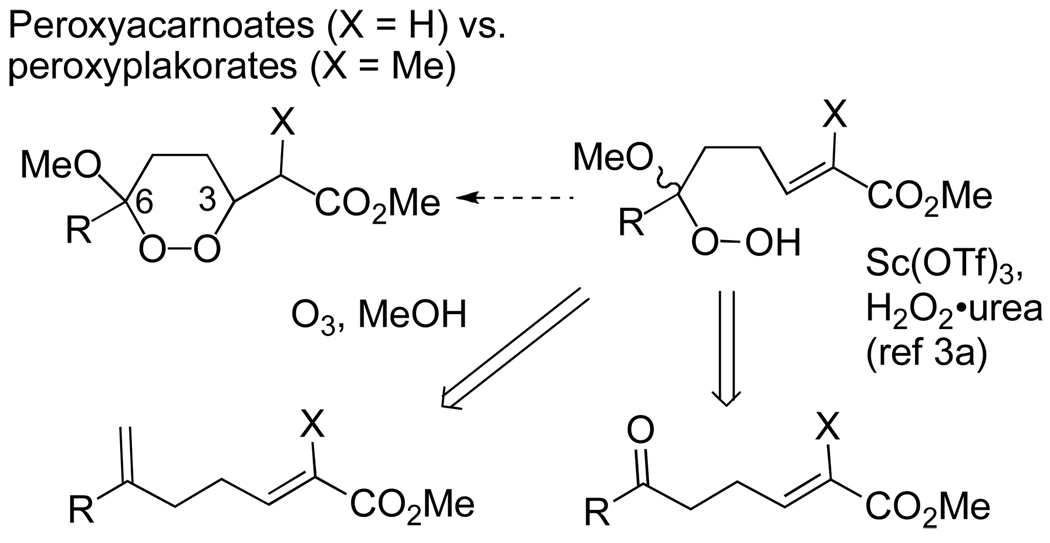
Most reported methods for the synthesis of 1,2-dioxanes involve electrophilic or radical cyclizations9,10,11 and are, in general, poorly suited for an approach to the peroxyplakorates. However, the 1,2-dioxane propionate core can be formed through conjugate addition of a hydroperoxy enoate. This reaction, first observed by Bartlett and coworkers during investigations of intramolecular nucleophilic epoxidations,12 was subsequently optimized for the synthesis of alkoxydioxanes,3 and has been applied by several groups to the synthesis of simplified peroxyplakorate analogs.4 However, a conjugate addition offers no obvious means of controlling the relative stereochemistry of the sidechain and dioxane stereocenters. 13 Furthermore, in the course of a recent total synthesis of structurally simpler peroxyacarnoates,8,14 we found that conjugate addition predominantly furnished the 1,2-dioxane isomer with C3 and C6 alkyl substituents in a trans (diequatorial) relationship; in the case of the peroxyplakorates, this would preclude any approach to the B-series of diastereomers. Finally, at the time these studies were initiated, conjugate addition offered no means for control of absolute stereochemistry, although a recent report on organocatalyst-mediated 5-exo-trig cyclizations of simple hydroperoxides offers some hope in this regard.15
The use of a conjugate addition also requires introduction of a hydroperoxyacetal nucleophile in presence of an enoate (Scheme 1). We found a reported acid-catalyzed peroxyacetalization3a to be very substrate dependent, something that has also been noted by others.4 Model studies suggested that selective ozonolysis of a C6-methylene, a reaction we had successfully employed in the presence of a disubstituted enoate (Scheme 1, X = H),14 was problematic in the presence of the trisubstituted enoate required for the peroxyplakorates (Scheme 1, X = CH3).
Scheme 1.
Methods for introduction of the hydroperoxyacetal
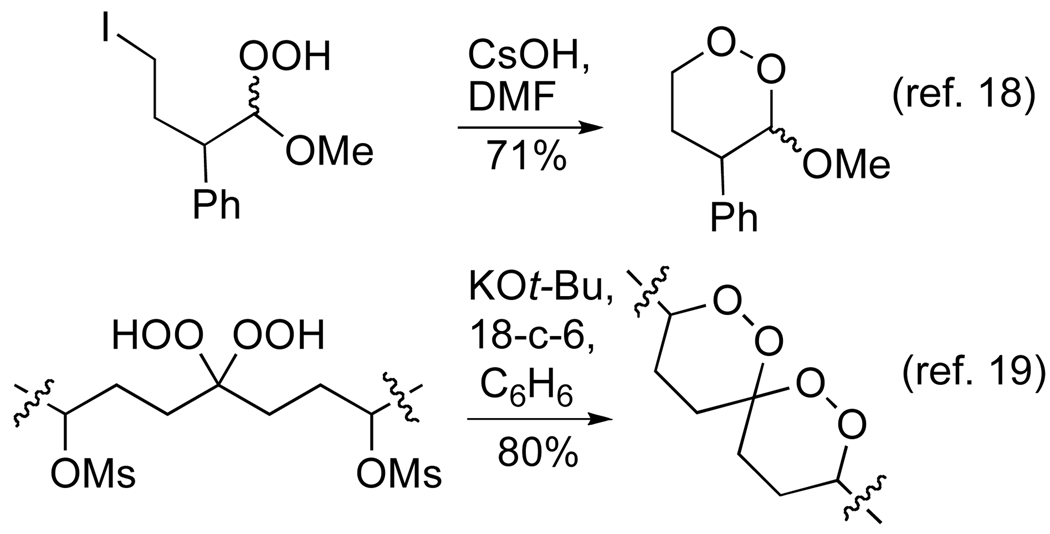
We became interested in a general approach to asymmetric synthesis of dioxanes based upon intramolecular nucleophilic substitution. Cyclization of a hydroperoxyepoxide has been applied to the asymmetric synthesis of the antimalarial Yingzhaosu A.16 However, the homologous opening of secondary oxetanes proved unsuccessful.17 These limitations led us to pursue intramolecular alkylations of hydroperoxyketals. We had previously observed the efficiency of intramolecular hydroperoxide alkylations in a 6-exo tet displacement of a primary iodide; 18 more recently, we employed a corresponding displacement of a secondary methanesulfonate as part of the synthesis of spiro bisperoxyacetals (Scheme 2).19
Scheme 2.
Intramolecular displacements with hydroperoxyacetals
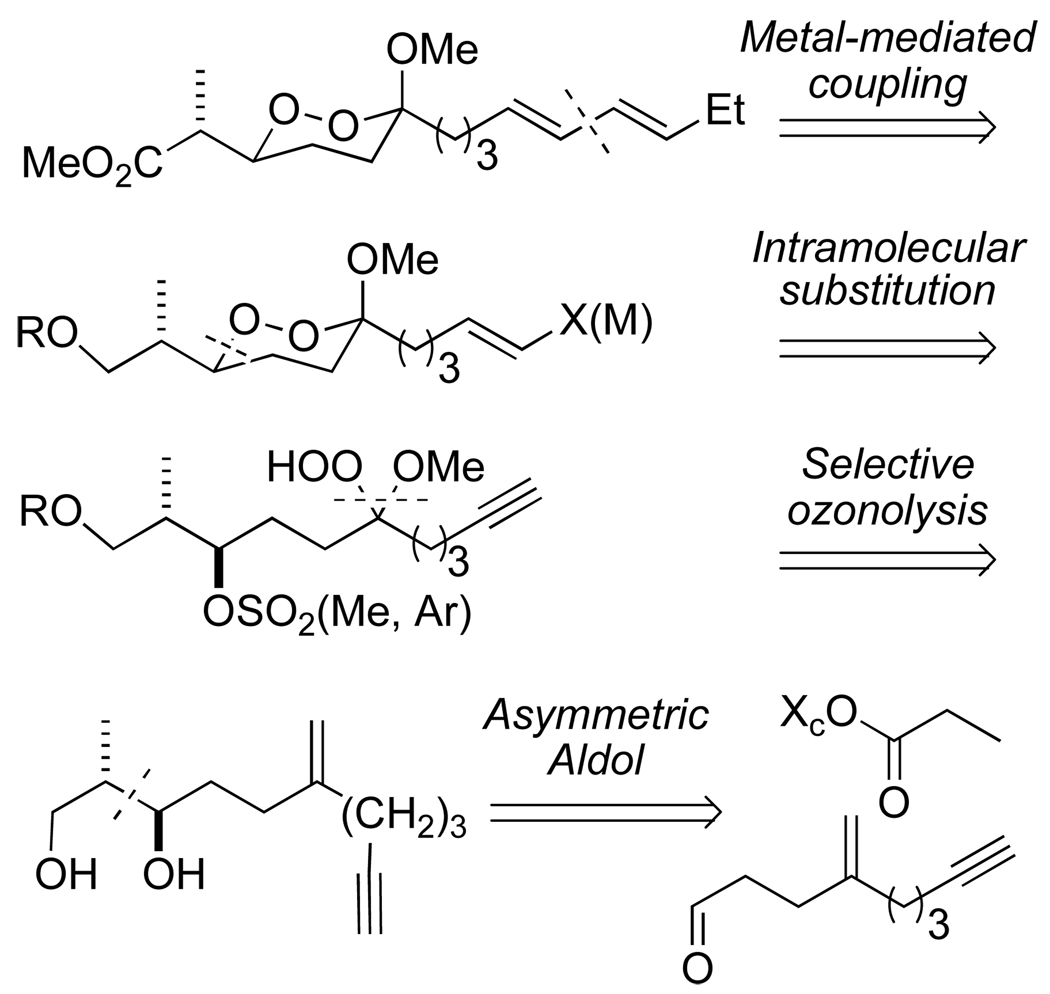
Our retrosynthetic approach (Scheme 3), illustrated for peroxyplakoric acid A3, is designed to allow a general solution to any member of the family. As the diene side chains in the peroxyplakorates are incompatible with ozonolytic introduction of the hydroperoxyacetal, we planned to employ Pd-mediated sp2/sp2 couplings as a disconnect to a metalloalkene or iodoalkene20 What remained to be seen was whether the functionalized alkene could be installed via hydrometallation of an alkynyl-substituted dioxane. The 1,2-dioxane ring would be installed through a stereospecific intramolecular displacement of a secondary sulfonate by a hydroperoxyacetal, which would in turn be revealed through ozonolysis of an alkene. Our previous work with the peroxyacarnoates had suggested that the terminal alkyne would be stable towards ozonolytic introduction of the hydroperoxyketal. 8,14 The secondary alcohol precursor of the sulfonate leaving group would be introduced via an asymmetric aldol reaction, with the choice of syn- or antiselective aldol determining the choice of peroxyplakoric A or B series.
Scheme 3.
Retrosynthesis of Peroxyplakorate A3
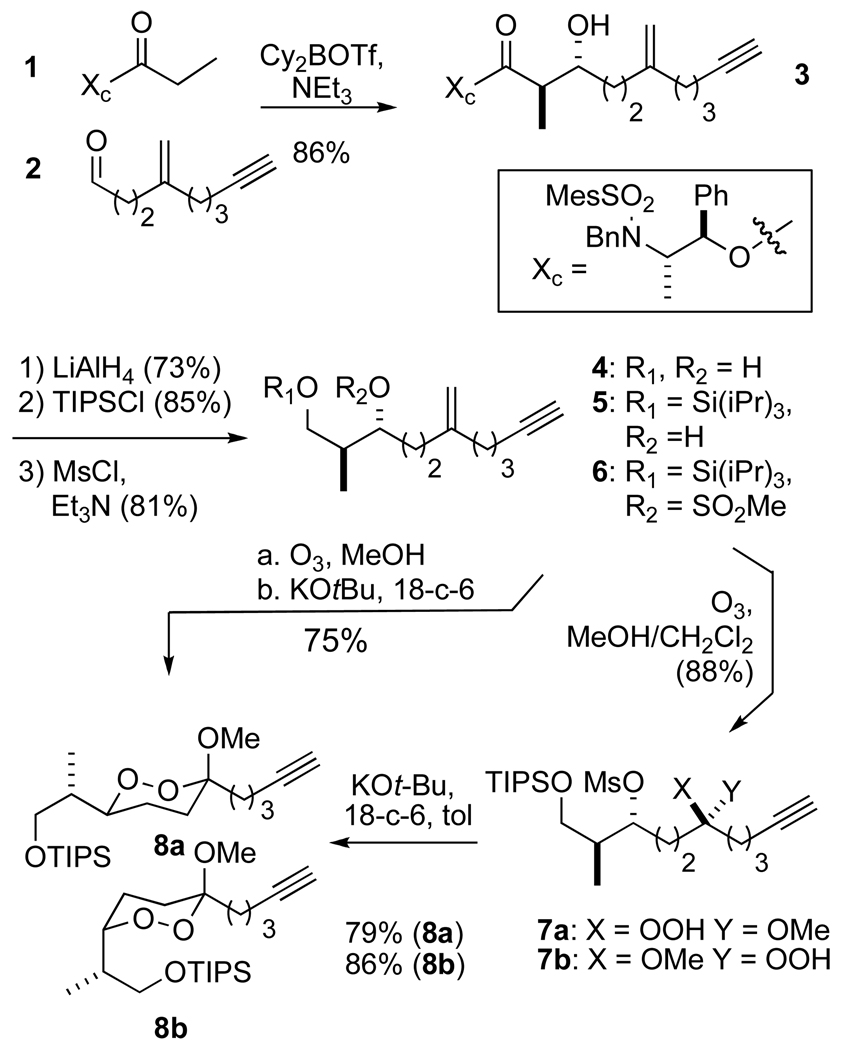
Synthesis of the functionalized 1,2-dioxane core is illustrated in Scheme 4. As our initial target, we chose the peroxyplakorate “A” series. The 2,3-syn stereochemistry requires an anti-aldol precursor. The dialkylboronate derived from chiral ester 121 underwent reaction with aldehyde 214 to furnish a good yield of a mixture of aldol products in which the desired (2R,3R)-anti diastereomer (3) predominated; the only significant byproduct (8%) was the syn-2S,3R-diasteromer. Reduction of 3 to the 1,3-diol 4 was followed by selective protection of the primary alcohol to form the triisopropyl silyl ether (5). Although attempted activation of the C3-hydroxyl as the triflate resulted in significant decomposition, the corresponding mesylate 6 was easily prepared. Methanolic ozonolysis of the methylene group furnished a high yield of a 1:1 mixture of hydroperoxyacetals 7a and 7b, which were incompletely separated even by semipreparative HPLC and therefore carried on as a mixture.22 Treatment with potassium tert-butoxide in the presence of 18-crown-6 furnished a 1:1 mixture of diastereomeric 1,2-dioxanes 8a and 8b in excellent yield. The configurational assignment of dioxane 8a was based upon a combination of precedent for the aldol reaction, as well as the 3JH constants for H3 (11.0, 6.3, 1.9 Hz) in the 1H NMR spectrum, which were very similar to the values reported for peroxyplakoric acids A1–A3 and consistent with typical observations for 1,2-dioxane natural products bearing an axial hydrogen at C3.2, 23 Interestingly, the undesired peroxyacetal 8b failed to epimerize in the presence of mild acid and was decomposed by strong acid (for example, methanolic HCl).
Scheme 4.
Dioxane core synthesis
Small quantities of the individual diastereomers 7a and 7b could be obtained by analytical HPLC. Subjecting the individual diastereomers to the cyclization conditions furnished the diastereomerically pure 1,2-dioxanes 8a and 8b, respectively, (Scheme 4) demonstrating that the cyclization was in fact stereospecific and allowing stereochemical assignment of the hydroperoxyacetals.
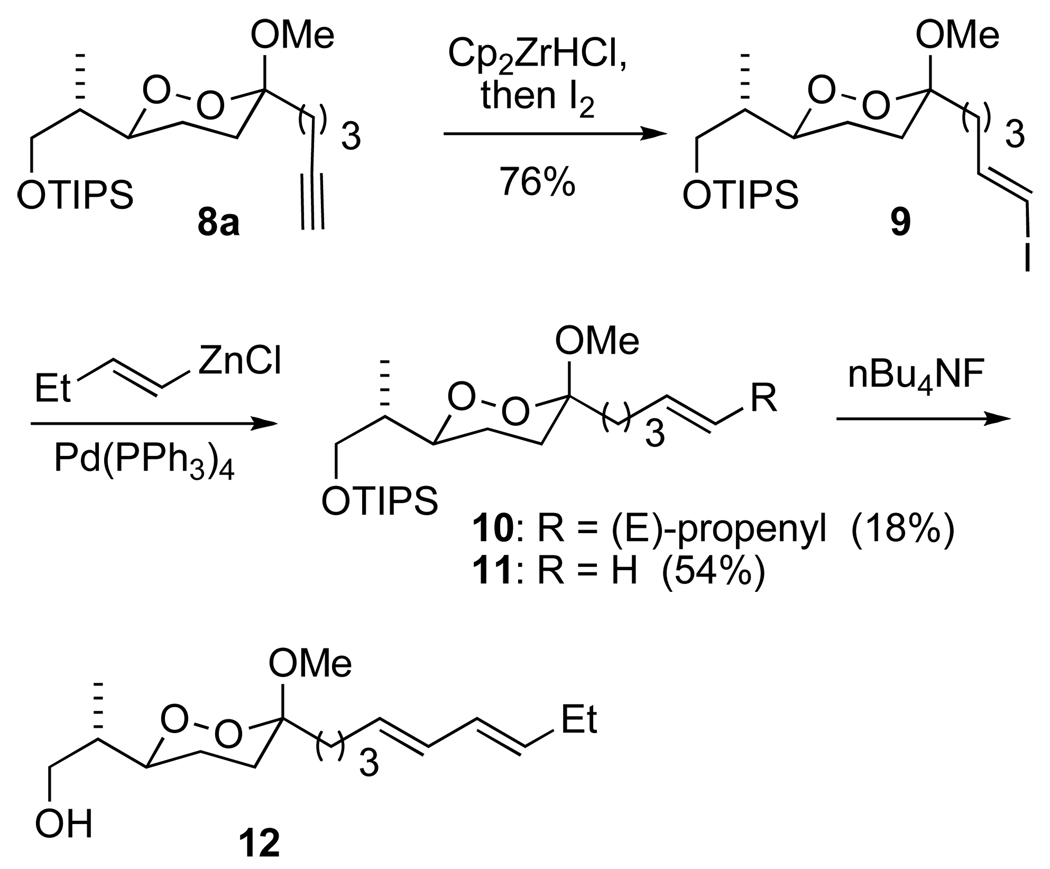
Chemoselective functionalization of the alkynyl dioxane to an E-iodoalkenyl dioxane was readily achieved by hydrozirconation with the Schwartz reagent, followed by quenching with iodine (Scheme 5). Our earlier studies had demonstrated the compatiblity of dialkyl peroxides with a number of Pd-mediated sp2/sp2 couplings,14,20,24 Cross coupling of the alkenyl iodide 9 with an E-butenyl zinc reagent derived from hydrozirconation of 1-butyne furnished a nearly inseparable 1:3 mixture of the desired diene and the terminal alkene derived from a net reduction.25 A small amount of diene, separated through careful chromatography, was treated with fluoride to reveal 12, the primary alcohol precursor of the peroxyplakorate A3.
Scheme 5.
Negishi coupling
3. Conclusion
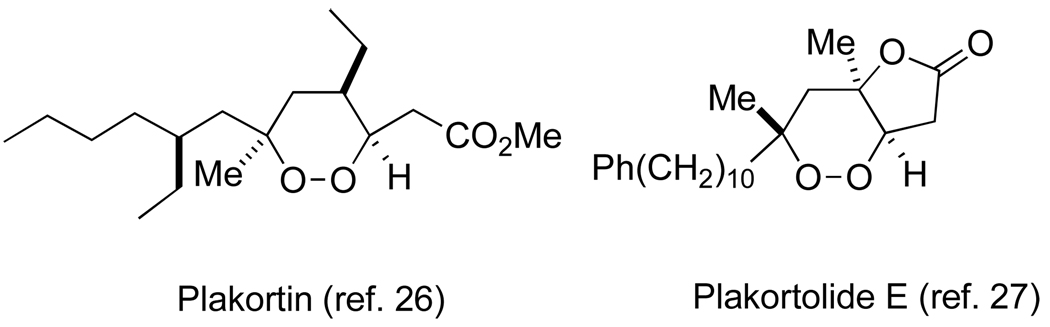
We have demonstrated an approach to the peroxyplakoric acid family of 1,2-dioxanes by a route, which, through choice of aldol precursor or the organometallic coupling partner, should be applicable to any member of the peroxyplakorates. The stereospecific intramolecular alkylation at the heart of the approach suggests a new and broadly applicable asymmetric approach to 1,2-dioxane natural products which include a secondary peroxide linkage; 1,6 two examples are shown below (Figure 2).26,27
Figure 2.
1,2-dioxanes with secondary peroxide elements
4. Experimental
4.1 General Procedures
Dichloromethane and THF were distilled from CaH2 and Na/benzophenone, respectively. All other reagents were used as received, except as noted. All reactions were conducted under an atmosphere of nitrogen except where noted. Thin layer chromatography (TLC) was performed on 0.25 mm hard-layer silica G plates; developed plates were visualized with UV lamp and/or by staining: 1% ceric sulfate and 10% ammonium molybdate in 10% H2SO4 (general stain, after charring); 1% N,N’-dimethyl-p-phenylenediamine solution in 1:20:100 acetic acid/water/methanol (specific for peroxides);28 1% aq. KMnO4 (for unsaturated compounds). “Standard drying and purification (solvent)” refers to drying of organic extracts over Na2SO4, removal of solvent under vacuum, and purification by flash chromatography using the indicated eluting solvent. Analytical HPLC was performed on a 4.6 mm × 25 cm Si column (5 µm); semi-preparative HPLC on a 21.4 mm × 25 cm Si column (8 µm); both employed RI detection. 1H, and 13C spectra were recorded at 300, 400, or 500 MHz in CDCl3 unless otherwise indicated; peaks are reported as: chemical shift (number of protons, multiplicity, J couplings in Hz). Infrared spectra were recorded as neat ATR films with selected absorbances reported in wavenumbers (cm−1). Optical rotations were measured on a digital polarimeter at ambient temperature.
4.2 (1R, 2S)-2-(N-Benzyl-N-mesitylenesulfonyl)amino-1-phenyl-1-propyl, propionate ester (1)
was prepared in three steps from norephedrine by a slight variant of the reported procedure in which the crude proprionate 1 was not triturated with hexane but directly recrystallized from EA/Hex.21
4.3 4-Methylenenon-8-ynal (2)
was prepared in three steps from 5-hexynoic acid by the method described in Xu, et al.14 The volatile and sensitive aldehyde was used without purification: 1H NMR (CDCl3) δ 9.6 (1 H, t, J = 1.6 Hz), 4.6-4.5 (2 H, m), 2.39 (2 H, dt, J = 1.6 Hz and 7.6 Hz), 2.1 (2 H, br t, J = 7.5 Hz), 1.99 (2 H, dt, J = 2.6 and 7.1 Hz), 1.94 (2 H, br t, J = 7.9 Hz), 1.8 (1H, t, J = 2.6 Hz), 1.5-1.4 (2 H, m but overall quintet J ~ 7.4 Hz); 13C NMR (CDCl3) δ 201.9, 146.6, 110.1, 84.0, 68.7, 41.7, 35.0, 27.9, 26.3, 17.8.
4.4 Dicyclohexyl(trifluoromethylsulfonyloxy)borane
was prepared by a slight modification of the method of Inoue, et al,21 in which the biphase obtained by allowing the crude reaction mixture to stand was separated by withdrawal of the lower phase with a syringe and needle.
4.5 (2R, 3R) [(1R, 2S)-2-(N-Benzyl-2,4,6-trimethylphenylsulfonamido)-1-phenylpropyl] 3-hydroxy-2-methyl-6-methyleneundec-10-ynoate (3)
was prepared from the chiral propionate 1 and aldehyde 2 by a variant of the method of Inoue, et al:21 Into an oven-dried 250 mL round bottom flask under nitrogen was added (1R, 2S)-2-(N-Benzyl-N-mesitylenesulfonyl) amino-1-phenyl-1-propyl propionate (3.1 g, 6.5 mmol) and 40 mL CH2Cl2. To this solution was added Et3N (2.2 mL, 16 mmol) via syringe. The solution was cooled to −78 °C and a solution of dicyclohexylboron triflate (1.0M in hexane, 13 mL, 13 mmol) was added dropwise over 15 minutes. The resulting solution was stirred at −78 °C for 2h. Crude 4-methylenenon-8-ynal (1.2 g, 7.9 mmol) in 5 mL CH2Cl2 was added dropwise to the enol boronate solution. The reaction mixture was stirred for 1 hr at −78 °C and was allowed to warm to room temperature over 1h, then quenched by addition of 0.1 M pH 7 phosphate buffer solution (24 mL). The mixture was diluted with 120 mL methanol, after which, 30% H2O2 (12 mL) was added carefully. The resulting suspension was stirred vigorously overnight and then concentrated. The residue was partitioned between water (50 mL) and CH2Cl2 (100 mL). The aqueous layer was extracted with CH2Cl2 (75 mL × 2). The combined organic layers were washed with water (60 mL × 3) and dried with Na2SO4. The filtered organic layer was concentrated and the residue was purified by flash chromatography using 15%–25% EA/Hex as the eluting solvent to afford 4 as a clear oil which solidified just below room temperature. Yield: 3.51 g, 86%; Rf = 0.33 (25% EA/Hex); [α]D = 19.5 (c 0.87, CHCl3); 1H NMR (CDCl3) δ 1.15 (3H, d, J = 7.3Hz), 1.19 (3H, d, J = 7.0 Hz), 1.49–1.69 (4H, m), 1.95 (1H, t, J = 2.6 Hz), 2.03–2.25 (4H, m), 2.18 (2H, td, J = 7.1 and 2.6 Hz), 2.28 (3H, s), 2.43–2.52 (1H, m), 2.49 (6H, s), 2.55 (1H, d, J = 6.8 Hz), 3.61–3.67 (1H, dtd, J = 3.5, 6.8, 9.5 Hz), 4.11–4.15 (1H, dq, 4.0 and 6.9 Hz), 4.54 (1H, B of AB, JAB = 16.5 Hz), 4.75 (1H, A of AB, JAB = 16.5 Hz), 4.75 (2H, s), 5.86 (d, 1H), 6.87 (2H, s), 6.88–6.90 (2H, m), 7.17–7.30 (8H, m); 13C NMR (CDCl3) δ 174.5, 148.2, 142.6, 140.3, 138.4, 138.2, 133.4, 132.1, 128.5, 128.4, 128.0, 127.7, 127.2, 126.0, 109.9, 84.3, 78.3, 72.8, 68.5, 56.8, 48.2, 45.5, 35.0, 32.4, 31.8, 26.5, 23.0, 21.0, 18.0, 14.1, 13.5; IR: 3532, 3299, 3031, 2981, 2942, 1737, 1604, 1454, 1381, 1321, 1153, 1013, 858, 757, 699, 659; MS: HR-FAB: calcd. for C38H47NO5S [M+Li]+: 636.3334, Found: 636.3346.
4.7 (2S, 3R)-2-Methyl-6-methyleneundec-10-yne-1,3-diol (4)
To a stirred solution of the chiral ester 3 (2.6 g, 4.2 mmol) in THF (40 mL) was added lithium aluminum hydride (0.19 g, 5.0 mmol) at 0 °C. The reaction mixture was stirred at room temperature for 1h and then quenched by the careful addition of Na2SO4•10 H2O. The mixture was stirred vigorously for 30 minutes and filtered. The residue obtained upon concentration was resuspended in hexane (60 mL) and then filtered, resulting in recovery of the auxiliary alcohol. The residue obtained upon concentration of the filtrate was separated by chromatography with 1:1 ethyl acetate and hexane to afford the 1,3-diol 5 as an clear colorless oil. Yield: 0.64 g, 73%; Rf = 0.27 (50%EA/Hex); [α]D = 31.7 (c 1.1, CHCl3); 1H NMR (CDCl3) δ 4.79 (1H, s), 4.77 (1H, s), 3.77 (1H, dd, J = 3.7 and 10.8 Hz), 3.55-3.64 (2H, m), 2.90 (2H, br s), 2.04–2.22 (6H, m), 1.95 (1H, t, J = 2.6 Hz), 1.53–1.77 (5H, m), 0.89 (3H, d, J = 7.0 Hz); 13C NMR (CDCl3) δ 148.6, 109.7, 84.3, 76.9, 68.5, 67.5, 39.8, 34.8, 33.2, 31.7, 26.4, 17.9, 13.8; IR: 3302 (br), 2939, 1644, 1432, 1275, 1026; MS: HR-FAB: calcd. for C13H22O2:[M+Li]+: 217.1780, Found: 217.1778.
4.8 (2S, 3R)-2-Methyl-6-methylene-1-(triisopropylsilyloxy)undec-10-yn-3-ol (5)
Diol 4 (0.55 g, 2.6 mmol) was dissolved in CH2Cl2 (15 mL) and cooled to 0 °C. To this solution was added Et3N (0.44 mL, 3.2 mmol), DMAP (32 mg, 0.26 mmol), and triisopropylsilyl chloride (0.61 mL, 2.9 mmol) sequentially. This mixture was warmed to room temperature and stirred for 15h, and then quenched with saturated aq. NH4Cl. The aqueous phase was extracted with Et2O, and the combined organic extracts were dried with Na2SO4, filtered, and concentrated in vacuo. The resulting crude material was purified by column chromatography using 5% EA/Hex to afford 0.81 g of the silyl ether as colorless oil. Yield 85%; Rf =0.44 (10% EA/Hex); [α]D = 25.1 (c 1.2, CHCl3); 1H NMR (CDCl3) δ 0.85 (3H, d, J = 7.0 Hz), 1.03–1.15 (21H), 1.49–1.76 (5H), 1.92 (1H, t, J = 2.6 Hz), 2.02–2.29 (6H, m), 3.54 (1H, m), 3.66 (1H, dd, J = 7.7 and 9.9 Hz), 3.88 (1H, dd, J = 3.9 and 9.9 Hz), 3.97 (1H, d, 3.0 Hz), 4.72 (1H, s), 4.76 (1H, s); 13C NMR (CDCl3) δ 148.9, 109.2, 84.4, 76.5, 69.1, 68.3, 39.6, 35.1, 33.4, 31.5, 26.5, 17.94, 17.89, 13.7, 11.7; IR: 3439 (br), 3314, 2943, 2886, 1645, 1463, 1386, 1276, 1261, 1062, 996, 883, 797; MS: HR-FAB: calcd. for C22H42O2Si:[M+H]+: 367.3033, Found: 367.3042.
4.9 (2S,3R)-2-Methyl-6-methylene-1-(triisopropylsilyloxy)undec-10-yn-3-yl methanesulfonate (6)
Alcohol 5 (0.70 g, 1.9 mmol) was dissolved in CH2Cl2 (10 mL) and the solution was cooled to 0 °C. Et3N (0.32 mL, 2.3 mmol) and MsCl (0.18 mL, 2.3 mmol) were added and the reaction mixture was allowed to warm to room temperature. After stirring for 14 h, the reaction mixture was diluted with CH2Cl2 and then washed with sat. aq. NH4Cl and brine and dried with Na2SO4. The crude mesylate obtained upon concentration was purified by chromatography (10% EA/Hex) to afford 0.69 g of mesylate 6. Yield: 0.69 g, 81%; Rf = 0.26 (10% EA/Hex); [α]D = 7.0 (c 0.97, CHCl3); 1H NMR (CDCl3) δ 0.97 (3H, d, J = 7.0 Hz), 1.04–1.11 (21H), 1.62–1.1.70 (2H), 1.77–1.89 (2H), 1.94 (1H, t, J = 2.6 Hz), 2.03–2.27 (7H), 3.00 (3H, s), 3.63 (1H, s), 3.65 (1H, s), 4.78 (2H, s), 4.89 (1H, dt, J = 3.9 and 8.8 Hz); 13C NMR (CDCl3) δ 147.5, 110.1, 85.1, 84.2, 68.5, 65.0, 39.7, 38.6, 34.9, 31.6, 28.4, 26.5, 18.0, 17.7, 11.9, 11.6; IR: 3312, 2943, 2893, 2866, 1646, 1463, 1336, 1175, 1109, 908, 884, 795, 678.; MS: HR-FAB: calcd. for C23H44O4SSi:[M+H]+: 445.2809, Found:445.2805.
4.10 6-Hydroperoxy-6-methoxy-2-methyl-1-(triisopropylsilyloxy)undec-10-yn-3-ylmethanesulfonate (7a and 7b)
A stream of O3/O2 was bubbled into a −78 °C solution of mesylate 7 (0.82 g, 1.8 mmol) in 15 mL methanol and 50 mL CH2Cl2 until the starting material had disappeared (TLC). The reaction was sparged with N2 and then allowed to warm to room temperature and concentrated in vacuo. The residue was purified through a short flash column using 30% EA/Hex to afford 0.77 g (88%) of a 1:1 mixture of diastereomers 7a and 7b. Yield: 0.77 g, 88%. Rf = 0.27 (33% EA/Hex). 13C NMR (CDCl3) δ 108.2, 108.0, 84.8, 84.3, 83.8, 83.7, 77.2,77.3, 69.0, 68.9, 48.7, 48.6, 39.6, 39.2, 38.7, 38.5, 29.6, 29.0, 27.0 24.8 24.6, 22.61, 22.60, 18.41, 18.40, 18.0, 12.1, 11.9, 11.8; HRFABMS calcd. for C23H46O7SSi: [M-HO2]+: 461.2675, Found: 461.2765.
Small quantities of pure diastereomers could be separated by analytical HPLC (EA/Hex):22 7a (2S, 3R, 6R); 1H NMR (CDCl3) δ 7.61 (1H, s), 4.87–4.91 (1H, m), 3.68 (1H, s), 3.66 (1H, s), 3.32 (3H, s), 3.04 (3H, s), 2.18–2.28 (3H, m (contains 2H, dt, J = 2.6 and 6.7 Hz at 2.27 ppm)), 1.99 (1H, t, J = 2.6Hz), 1.90–1.98 (1H, m), 1.72–1.82 (5H, m), 1.50–1.63 (3H, m), 1.03–1.14 (21H, m), 0.98 (3H, d, J = 7.0Hz). 7b (2S, 3R, 6S); 1H NMR (CDCl3) δ 8.08 (1H, s), 4.91–4.95 (1H, m), 3.69 (1H, s), 3.68 (1H, s), 3.34 (3H, s), 3.05 (3H, s), 2.14–2.28 (3H, m (contains 2H, dt, J = 2.6 and 6.7 Hz at 2.26 ppm)), 1.88–2.0 (3H, m (contains 1H, t, J = 2.6 Hz)), 1.66–1.80 (4H, m), 1.50–1.63 (3H, m), 1.03–1.14 (21H, m), 0.98 (3H, d, J = 7.0 Hz).
4.11 (2S)-1-Triisopropylsiloxy-2-((3S, 6R)-6-methoxy-6-(pent-4-ynyl)-1,2-dioxan-3-yl) propane (8a)
To a solution of hydroperoxy acetal (7a) (17.3 mg, 0.03 mmol) in anhydrous toluene (0.4 mL) was added 18-crown-6 (9.5 mg, 0.04 mmol), followed by KOt-Bu (5.8 mg, 0.05 mmol). The reaction mixture was stirred for 20 minutes, at which point additional KOt-Bu (3.3 mg, 0.03 mmol) was added. After stirring for an additional 20 min, the reaction became yellow, and TLC indicated the absence of starting material. The crude reaction mixture was filtered through a short pad of silica gel with 20% EA/Hex. The filtrate was concentrated and the residue was purified by column chromatography using 5% EA/Hex to afford dioxane 8a. Yield:11.3 mg, 79%; Rf = 0.36 (5%EA/Hex); [α]D = −144 (c 0.74, CHCl3); 1H NMR (CDCl3): δ 4.05 (1H, ddd, J = 1.9, 6.3, 11.0 Hz), 3.68 (1H, dd, J = 5.2 and 9.8 Hz), 3.63 (1H, dd, J = 5.6 and 9.8Hz), 3.28 (3H, s), 2.19–2.24 (2H, m), 1.96 (1H, t, J = 2.6 Hz), 1.41– 1.92 (9H, m), 1.03–1.13 (21H, m), 1.01 (3H, d, J = 6.9Hz); 13C NMR (CDCl3): δ 102.3, 83.8, 81.7, 68.8, 65.1, 48.4, 39.7, 31.9, 30.7, 22.9, 22.1, 18.5, 18.0, 12.7, 12.0; IR: 3312, 2943, 2866, 1462, 1238, 1109, 1066, 917, 882, 789, 746, 682; MS: HR-FAB: calcd. for C22H42O4Si:[M+Li]+ 405.3012, Found: 405.3000.
4.12 (2S)-1-Triisopropylsiloxy-2-((3S, 6S)-6-methoxy-6-(pent-4-ynyl)-1,2-dioxan-3-yl) propane (8b)
By a similar procedure, hydroperoxy acetal (7b) (20 mg, 0.04 mmol) was converted to 1,2-dioxane 8b. Yield: 14.5 mg, 87%; Rf = 0.36 (5%EA/Hex); [α]D = +121.4 (c 1.4, CHCl3); 1H NMR (CDCl3) δ 3.93 (1H, dt, J = 8.5 and 5.7 Hz), 3.70 (1H, dd, J = 4.7 and 9.9 Hz), 3.67 (1H, dd, J = 4.7 and 8.8 Hz), 3.34 (3H, s), 2.25 (2H, dt, 2.6 and 6.7 Hz), 2.03–2.15 (2H, m), 1.99 (1H, t, J = 2.6 Hz), 1.75–1.86 (2H, m), 1.54–1.74 (5H, m), 1.12 (3H, d, J = 6.8 Hz), 1.04–1.10 (21H, m); 13C NMR (CDCl3) δ 102.3, 83.8, 81.7, 68.8, 65.1, 48.4, 39.7, 31.9, 30.7, 22.9, 22.1, 18.5, 18.0, 12.7, 12.0; IR: 3312, 2943, 2866, 1462, 1238, 1109, 1066, 917, 882, 789, 746, 682; MS: HR-FAB: calcd. for C22H42O4Si:[M+Li]+ 405.3012, Found: 405.3000.
4.13 One Pot Ozonolysis/Cyclization Method: (2S)-1-Triisopropylsiloxy-2-((3S, 6RS)-6-methoxy-6-(pent-4-ynyl)-1,2-dioxan-3-yl)propane (8ab)
Mesylate 6 (0.6811 g, 1.5 mmol) was dissolved in 20 mL CH2Cl2 and methanol (7.8 mL, 193 mmol). The solution was cooled to −78 °C and treated with a stream of O3/O2. The reaction was monitored by TLC until starting material was absent. The reaction mixture was then sparged with nitrogen and allowed to warm to room temperature. To this solution was added 18-Crown-6 (396.8 mg, 1.5 mmol), followed by KOt-Bu (154.2 mg, 1.4 mmol). The reaction mixture was stirred for 20 minutes, at which point additional KOt-Bu (170.5 mg, 1.5 mmol) was added. After stirring for an additional 20 min, the reaction became yellow, and TLC indicated the absence of starting material. The crude reaction mixture was filtered through a short pad of silica gel with 20% EA/Hex. The filtrate was concentrated and the residue was purified by column chromatography using 5% EA/Hex to afford 458.4 mg (75%) of an approximately 1:1 mixture of 8a and 8b. Yield: 75%.
4.14 (2S)-1-Triisopropylsiloxy-2-((3S,6R)-6-methoxy-6-((4E)-4-iodopent-4-enyl)-1,2-dioxan-3-yl) propane (9)
1,2-Dioxane 8a (0.43 g, 1.1 mmol), azeotropically dried by concentration from distilled toluene (2×15 mL), was dissolved in 10 mL dry toluene. To this solution was added Cp2ZrHCl (0.38 g, 1.5 mmol) under a nitrogen atmosphere. After stirring for 15 minutes, the solution became light orange in color. Another portion of Cp2ZrHCl (0.21 g, 0.8 mmol) was added and the reaction mixture was stirred for another 15 minutes. Iodine (0.41 g, 1.6 mmol) was added slowly until the dark red color became permanent. The reaction mixture was diluted with 50 mL Hex, and filtered through Celite. The filtrate was washed with saturated aqueous Na2S2O3, dried with Na2SO4 and concentrated. The residue was purified by column chromatography using 5–10% EA/Hex to afford 0.43 g (76%) of a 1:3 mixture of the terminal alkene reduction product (due to water) and the desired terminal E-iodoalkene 9 which could be separated by semi-prep HPLC (EA/Hex). Rf = 0.32 (5% EA/Hex); [α]D = −97.2 (c 0.94, CHCl3); 1H NMR (CDCl3) δ 6.48 (1H, dt, J = 14.4 and 7.1 Hz), 6.01 (1H, dt, J = 14.4 and 1.3 Hz), 4.01–4.06 (1H, m), 3.67 (1H, dd, J = 5.2 and 9.9 Hz), 3.63 (1H, dd, J = 5.6 and 9.9 Hz), 3.24 (3H, s), 2.03–2.08 (2H, m), 1.82–1.92 (2H, m), 1.24–1.67 (7H, m), 1.03–1.12 (21H, m), 1.00 (3H, d, J = 6.9 Hz); 13C NMR (CDCl3) δ 145.7, 102.3, 81.7, 75.2, 65.0, 48.4, 39.6, 36.0, 32.1, 30.7, 22.9, 21.8, 18.0, 12.7, 11.9; IR: 2942, 2865, 1462, 1234, 1110, 1070, 1030, 946, 882, 788, 683, 659; MS: HRFAB: calcd. for C22H43LiO4Si:[M+Li]+ 533.2136 , Found: 533.2125.
4.11 (2S)-1-Triisopropylsiloxy-2-((3S,6R)-6-methoxy-6-((4E,6E)-nona-4,6-dienyl)-1,2-dioxan-3-yl) propane (10)
1-Butyne (0.12 g, 2.2 mmol) was condensed into a 50 mL flask at −78 °C under a N2 atmosphere, and dissolved in distilled THF (4 mL) and distilled toluene (4 mL). The solution was warmed slowly to 0 °C, whereupon Cp2ZrHCl (0.22 g, 0.85 mmol) was added. The reaction was held at 0 °C for one hour, after which another portion of Cp2ZrHCl (0.10 g, 0.39 mmol) was added. The reaction mixture was stirred for another hour at 0 °C then the ice bath was then removed. After 10 minutes, the solution was cooled to 0 °C again and ZnCl2 (0.12 g, 0.88 mmol) in 2 mL dry THF was added.25 The reaction solution turned orange and was stirred at 0 °C for 10 minutes. Vinyl iodide 9 (0.16 g, 0.30 mmol) was added, followed by Pd(PPh3)4 (45 mg, 0.04 mmol). The reaction was stirred at 0 °C for 5 minutes. TLC indicated the presence of significant amount of starting material, and another portion of Pd(PPh3)4 (15 mg, 0.01 mmol) was added. The reaction mixture was stirred at 0 °C for an additional 0.5 h, at which point TLC indicated the absence of starting material. The reaction was diluted with 20 mL hexane, and the reaction mixture was filtered through a short pad of silica gel with 30% EA/Hex. The filtrate was concentrated in vacuo and the residue was purified by flash chromatography (5% EA/Hex) to give 0.13 g of an inseparable mixture of diene 10 and terminal alkene 11. The coupled and reduced products were separable after removal of the TIPS ether (next step).
4.12 ((2S)-2-((3S, 6R)-6-Methoxy-6-((4E, 6E)-nona-4,6-dienyl)-1,2-dioxan-3-yl) propan-1-ol (12)
A few mg of the mixture of diene and terminal alkene from the previous step was dissolved in THF. A solution of TBAF (nominally 1M in THF) was added dropwise until TLC indicated the disappearance of the starting material. The reaction mixture was washed with saturated ammonium chloride solution and water sequentially. Then the separated organic layer was dried with Na2SO4, concentrated. The residue was first purified by flash chromatography, and then by HPLC. The desired deprotected diene product was very easily isolated by HPLC. 1H NMR (CDCl3): δ 5.95–6.06 (2H, m), 5.49–5.66 (2H, m), 4.10–4.14 (1H, m), 3.54–3.70 (m, 2H), 3.25 (3H, s), 2.04–2.20 (4H, m), 1.82–2.01 (3H, m), 1.25–1.72 (6H, m), 0.99 (3H, t, J = 7.4 Hz), 0.96 (3H, d, J = 7.2 Hz); 13C δ 134.5, 131.2, 131.0, 129.1, 102.8, 82.4, 65.1, 48.4, 39.1, 32.5, 32.2, 30.5, 25.6, 22.7, 21,6, 13.6, 11.9; HRFABMS calcd. for C17H30O4Li:[M+Li]: 305.2304; Found: 305.2305 (0.2 ppm).
Acknowledgement
Research was conducted in facilities remodeled with support from NIH (RR016544-01). NMR spectra were acquired, in part, on spectrometers purchased with NSF support (MRI 0079750 and CHE 0091975). We thank Dr. Don Davies and Ms. Laure Chauset for some initial experiments related to alkylation of hydroperoxyacetals, and Ms. Carita Kordik for assistance with mass spectrometry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Casteel DA. Nat. Prod. Reports. 1999;16:55. [Google Scholar]
- 2.Kobayashi M, Kondo K, Kitagawa I. Chem. Pharm. Bull. 1993;41:1324. doi: 10.1248/cpb.41.1324. [DOI] [PubMed] [Google Scholar]
- 3.a. Murakami N, Kawanishi M, Itagaki S, Horii T, Kobayashi M. Tetrahedron Lett. 2001;42:7281. [Google Scholar]; b. Kawanishi M, Kotoku N, Itagaki S, Horii T, Kobayashi M. Bioorg. Med. Chem. 2004;12:5297. doi: 10.1016/j.bmc.2004.04.051. and references within. [DOI] [PubMed] [Google Scholar]
- 4.For recent applications of conjugate addition for the conjugate addition of hydroperoxyacetals to enoates, see: Jin H-X, Zhang Q, Kim H-S, Wataya Y, Liu H-H, Wu Y. Tetrahedron. 2006;62:7699. Liu H-H, Jin H-X, Zhang Q, Wu Y-K, Kim H-S, Wataya Y. Chin. J. Chem. 2005;23:1469..
- 5.For leading references to synthesis of some other classes of 6-membered ring peroxide natural products, see: Jung M, Ham J, Song J. Org. Lett. 2002;4:2763. doi: 10.1021/ol026285x. Yao G, Steliou K. Org. Lett. 2002;4:485. doi: 10.1021/ol016943y. Snider BB, Shi ZJ. Am. Chem. Soc. 1992;114:1790. Dussault PH, Eary CT, Woller KR. J. Org. Chem. 1999;64:1789. doi: 10.1021/jo981128q..
- 6.For the asymmetric synthesis of an alkoxydioxane via chemoselective hydrogenation of a 1,2-dioxine, see: Dussault PH, Kreifels S, Lee IQ. Synth. Commun. 1995;25:2613..
- 7.Casteel DA. Nat. Prod. Reports. 1992;9:289. doi: 10.1039/np9920900289. [DOI] [PubMed] [Google Scholar]
- 8.Portions of this work have been reported: Xu C. Total Synthesis of Peroxyacarnoic Acids and Peroxyplakoric Acids. New Synthetic Methodology for Organic Peroxides and Application of Peroxides as Enzyme Inhibitors. University of Nebraska-Lincoln, ISBN 97805426543510; 2006. .
- 9.For leading references to synthesis of peroxide heterocycles, see; Korshin E, Bachi MD. In: Chemistry of Peroxides. Rappoport Z, editor. v. 2. Chichester: John Wiley & Sons; 2006. pp. 189–305..
- 10.For leading references to radical promoted synthesis of peroxide heterocycles, see; Nishino H. Topics Heterocyclic Chem. 2006;6:39..
- 11.For leading references to electrophilic cyclizations, see; Ref. 9 Harris JR, Waetzig SR, Woerpel KA. Org. Lett. 2009;11:3290. doi: 10.1021/ol901046z. Ushigoe Y, Kano Y, Nojima M. J. Chem. Soc., Perkin Trans. 1. 1997:5. Dussault PH, Davies DR. Tetrahedron Lett. 1996;37:463..
- 12.Bartlett PA, Chapuis C. J. Org. Chem. 1986;51:2799. [Google Scholar]
- 13.Previous approaches to peroxyplakoric analogs have focused on targets lacking the exocyclic stereocenter: see references 3b and 4.
- 14.Xu C, Raible JM, Dussault PH. Org. Lett. 2005;7:2509. doi: 10.1021/ol050291m. [DOI] [PubMed] [Google Scholar]
- 15.Lu X, Liu Y, Sun B, Cindric B, Deng L. J. Am. Chem. Soc. 2008;130:8134. doi: 10.1021/ja802982h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X-X, Dong H-Q. J. Org. Chem. 1995;60:3039. [Google Scholar]
- 17.Dai P, Dussault PH. Org. Lett. 2005;7:4333. doi: 10.1021/ol051407h. [DOI] [PubMed] [Google Scholar]
- 18.Dussault PH, Zope UR. J. Org. Chem. 1995;60:8218. [Google Scholar]
- 19.Ghorai P, Dussault PH, Hu C. Org. Lett. 2008;10:2401. doi: 10.1021/ol800657m. [DOI] [PubMed] [Google Scholar]
- 20.Dussault PH, Eary CT, Lee RJ, Zope UR. J. Chem. Soc., Perkin Trans. 1. 1999:2189. [Google Scholar]
- 21.Inoue T, Liu J-F, Buske DC, Abiko A. J. Org. Chem. 2002;67:5250. doi: 10.1021/jo0257896. [DOI] [PubMed] [Google Scholar]; Abiko A. Acc. Chem. Res. 2004;37:387. doi: 10.1021/ar030249w. [DOI] [PubMed] [Google Scholar]; Abiko A. Org. Synth. 2003;79:116. [Google Scholar]
- 22.Hydroperoxyketals 7a and 7b are relatively labile. While we were sometimes able to separate small amounts of individual epimers, extensive chromatography more often resulted in partial decomposition to form the corresponding ketone.
- 23.See, for example, Sperry S, Valeriote FA, Corbett TH, Crews P. J. Nat. Prod. 1998;61:241. doi: 10.1021/np970467w..
- 24.Dussault PH, Eary CT. J. Am. Chem. Soc. 1998;120:7133. [Google Scholar]
- 25.Wipf P, Nunes RL. Tetrahedron. 2004;60:1269. [Google Scholar]
- 26.Plakortin: Higgs MD, Faulkner DJ. J. Org. Chem. 1978;43:3454. Cafieri F, Fattorusso E, Taglialatela-Scafati O, Ianaro A. Tetrahedron. 1999;55:7045..
- 27.Plakortolide E: Varoglu M, Peters BM, Crews P. J. Nat. Prod. 1995;58:27. doi: 10.1021/np50115a003..
- 28.Smith L, Hill FL. J. Chrom. 1972;66:101. doi: 10.1016/s0021-9673(01)82933-2. [DOI] [PubMed] [Google Scholar]