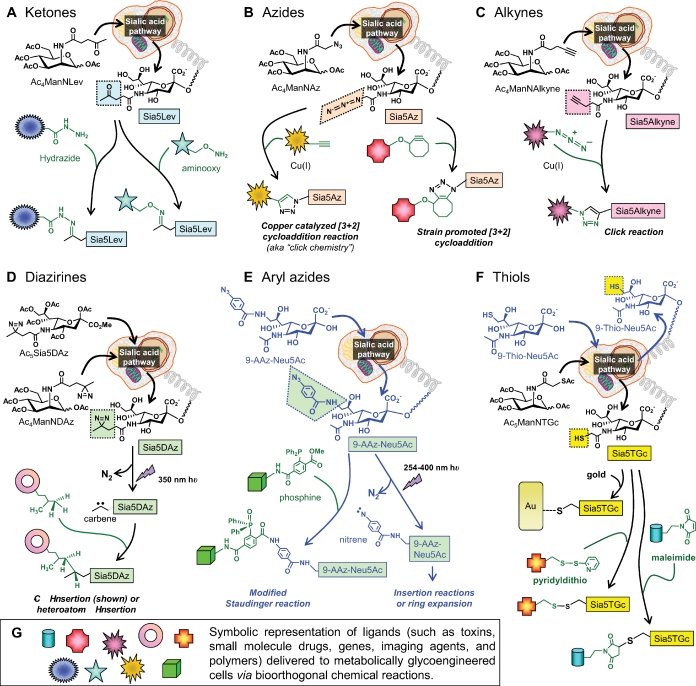
Fig. 5.
Bioorthogonal ligation reactions, exemplified by glycan-displayed ketones, azides, alkynes, diazirines, aryl azides, and thiols. (A) Ketones displayed on cells incubated with ManNLev or Ac4ManNLev (Jacobs et al. 2001) can be ligated with hydrazide in a pH-dependent reaction (Yarema et al. 1998) or with aminooxy in a pH-independent reaction (Nauman and Bertozzi 2001). (B) Azides can be displayed in sialosides using Ac4ManNAz and ligated with phosphines using the Bertozzi-modified Staudinger reaction (shown for aryl azides in (E) (Saxon and Bertozzi 2000) or via [3+2] cycloaddition with alkynes using copper-catalyzed click reactions or strain-promoted cyclootyne (Agard et al. 2004). (C) Conversely, alkynes can be displayed in glycans (e.g., by using Ac4ManNAlkyne) and ligated with azide-derivatized ligands or fluorophores (Hsu et al. 2007). (D) The diazirine-containing analog Ac4ManNDAz (or Ac5Sia5DAz) installs photoactivatable Sia5DAz onto the cell surface where irradiation produces carbenes that undergo insertion reactions with neighboring biological molecules (Tanaka and Kohler 2008), thus crosslinking in situ sialoside ligands. (E) Aryl azides can be incorporated into sialic acid via 9-AAZ-Neu5Ac, which can then be subject to the modified Staudinger reaction referenced in (B) or irradiated to provide crosslinking reactions similar to those resulting from diazirine in (D) (Han et al. 2005). (F) Thiols can be incorporated into sialosides using Ac5ManNTGc (Sampathkumar, Li, Jones, et al. 2006) or 9-thio-Neu5Ac (Oetke et al. 2002), which can then bind to gold or undergo the chemoselective reaction with pyridyldithio- or maleimide-derivatized ligands. (G) In this figure, ligands being delivered to a cell are represented symbolically with specific examples provided in the main text and references therein.