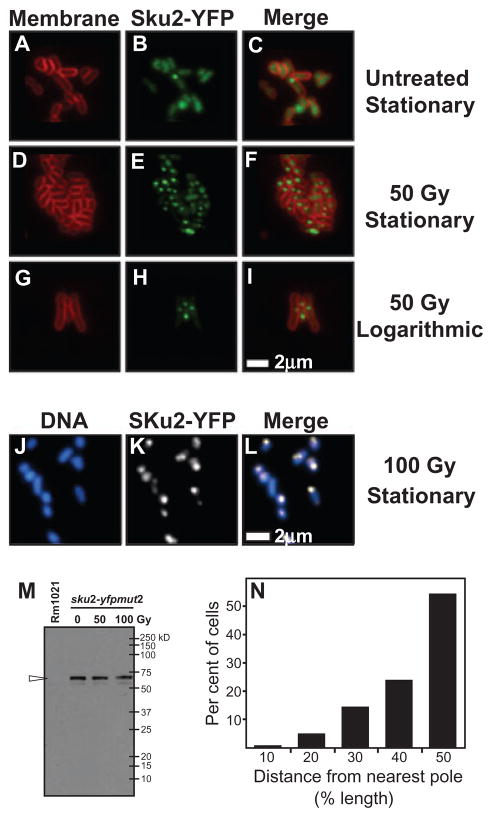
Fig. 4.
Subcellular localization of SKu2 in free-living S. meliloti cells. Panels A–L represent subcellular localization of SKu2–YFP fusion proteins in free-living S. meliloti by epi-fluorescence microscopy. Rm1021sku2-yfpmut2 was grown in M9 medium supplemented with succinate. Cells of the stationary phase (A–E and J–L) or the logarithmic phase (G–I) were irradiated by 50 Gy of IR (D–I), 100 Gy of IR (J–L) or mock-treated (A–C). Localization of SKu2-YFP was visualized in living cells as represented in green (B, E and H) or white (K). The cell membrane was stained by FM4-64 as represented in red (A, D and G). Cellular DNA was stained by DAPI as represented in blue (J). Epi-fluorescence views of different filters were merged to analyse relative localization (C, F, I and L). No focus was observed in cells of the Rm1021 wild-type strain (data not shown). Scale bar represents 2 μm. Panel M represents immunoblot analysis of SKu2-YFP protein levels in Rm1021sku2-yfpmut2 after treatment with the indicated level of IR. Cells of stationary phase were treated with IR of 0, 50 and 100 Gy. Whole-cell extracts were blotted onto PVDF membrane and probed with anti-GFP (Abcam). One milligram of total protein from each cell extracts was loaded. Although few signals of endogenous proteins cross-reacting with anti-GFP were detected commonly in extracts from both Rm1021sku2-yfpmut2 (sku2-yfpmut2) and the wild-type control (Rm1021), strong signals of SKu2-YFP, a protein of expected molecular weight of 60 kDa, were only detected in extracts of Rm1021sku2-yfpmut2 (indicated with a open arrow head). Panel N represents the subcellular position of SKu2-YFP as a percentage of cell length for single-focus cells (n = 95). Cells of stationary phase were treated with IR of 50 Gy. The relative position of SKu2-YFP foci in each cell was determined as a ratio (%) of the distance between foci and the proximal pole to the longitudinal length of the cell.