Abstract
Although frequency-domain analysis of heart rate variability (HRV) has been performed in the setting of exercise and recovery from exercise, the relationship of specific frequency components to sympathetic and parasympathetic inputs has not been validated in this setting. The aim of this study is to evaluate the relationship of frequency components of HRV to sympathetic and parasympathetic modulation in the setting of recovery after exercise using selective autonomic blockade. Normal subjects (n = 27, 17 men, 53 ± 7 yr old) underwent bicycle stress testing on four separate days. On day 1, a baseline study without autonomic blockade was performed. On days 2 through 4, either β-adrenergic, parasympathetic, or double blockade was administered during exercise and completed 3 min before recovery. Continuous ECG was recorded for 5 min starting from the end of exercise. Time- and frequency-domain measures of HRV were computed for each of the five 1-min segments of RR intervals. Parasympathetic blockade significantly decreased all the HRV measures compared with baseline (P < 0.02 for all). Root mean square of successive differences of RR intervals (rMSSD) was increased by β-adrenergic blockade (P < 0.0002). All the HRV measures except rMSSD showed increases with time after the first minute of recovery. The low frequency-to-high frequency ratio did not respond to autonomic blockade or to recovery time, consistent with the expected changes in sympathovagal influence. Root mean square (detrended SD) and rMSSD were highly correlated with the square root of the total power (r = 0.96) and high-frequency power (r = 0.95), respectively. Although there are marked reductions in the frequency-domain measures in recovery versus rest, the fluctuations in the low- and high-frequency bands respond to autonomic blockade in the expected fashion. Time-domain measures of HRV were highly correlated with frequency-domain measures and therefore provide a computationally more efficient assessment of autonomic influences during recovery from exercise that is less susceptible to anomalies of frequency-domain analysis.
Keywords: heart rate recovery, autonomic nervous system
early studies of heart rate variability (HRV) in the spectral domain have shown that the spectral power contained in certain frequency bands reflects, in part, the sympathetic and parasympathetic modulation of sinus node activity (1). Studies using selective autonomic blockade have shown that power in the frequencies of HRV > 0.15 Hz can be attributed to parasympathetic modulation, whereas power in frequencies < 0.15 Hz are related to both sympathetic and parasympathetic modulation (1, 27). Thus spectral analyses of HRV have been used in a variety of settings and pathologies to assess autonomic modulation (3, 4, 8, 11, 12, 20, 21, 36).
Rapid changes in sympathovagal control are known to occur in the setting of exercise and recovery from exercise. Exercise is characterized by a decrease in parasympathetic tone and an increase in sympathetic tone, resulting in an increase in heart rate (15, 23). During recovery from exercise, heart rate gradually decreases as parasympathetic tone returns and sympathetic tone withdraws (17, 30). Although HRV during recovery from exercise has been studied using frequency-domain measures, the validity of the results has not been formally tested (6, 24, 28, 31). Specifically, the initial autonomic blockade studies evaluating the parasympathetic contributions to the various spectral bands were performed at rest and with a change in position (tilt, standing); no such studies have been performed to evaluate whether the information contained in these same spectral bands during exercise and recovery correspond to the same physiology demonstrated at rest and with a change in position. With exercise, there are significant autonomic changes, a dramatic reduction in total spectral power, and a change in the respiratory rate. In fact, a number of studies have reported conflicting results when using spectral analysis to evaluate HRV during exercise and recovery (2, 3, 5, 25, 26, 29, 33, 37).
We therefore sought to validate the physiological origins of frequency-domain measures of HRV during recovery from exercise using selective autonomic blockade. If high-frequency (HF) components of HRV reflect parasympathetic modulation of RR intervals during recovery from exercise, we should expect a significant increase in HF power due to the parasympathetic reactivation during the first few minutes of recovery after exercise. Similarly, if the ratio of low-frequency (LF) and HF power reflects sympathovagal interactions (27), we should expect this ratio to be high at peak exercise and decrease during recovery. Selective autonomic blockade should either attenuate or abolish these responses. We further compared the spectral components to two previously validated time-domain measures of HRV (14).
METHODS
Exercise protocol.
Thirty-eight normal subjects were originally recruited for this study to evaluate the QT-RR interval relationship and HRV during recovery from exercise under the setting of selective autonomic blockade (35). Of these 38, 27 subjects (17 men, 53 ± 7 yr old) were able to complete bicycle stress testing on 4 separate days for 24 min with adequate ECG quality for each. The study protocol has been described previously in detail (35). Briefly, on day 1, a baseline study without autonomic blockade was performed. Workload was incremented during exercise to a maximum of 125 W, and this peak intensity was repeated for all subsequent stress tests. On days 2 and 3, either atropine (0.04 mg/kg) to achieve parasympathetic blockade or propranolol (0.2 mg/kg) to achieve β-adrenergic blockade was administered during exercise and completed 3 min before the start of recovery. On day 4, a double blockade with propranolol (0.2 mg/kg) and atropine (0.04 mg/kg) was administered during exercise. These doses of atropine and propranolol were shown by Jose and Collison (18) to achieve complete blockade. Oscillometric blood pressure measurements were taken at rest, peak exercise, and 5 min after the cessation of exercise for each test. All subjects were studied at the Clinical Research Center at Northwestern Memorial Hospital (Chicago, IL). Informed consent was obtained from all subjects before testing. The protocol was approved by the Institutional Review Board of Northwestern University.
ECG analysis.
Five minutes of continuous Frank-lead ECGs were recorded during rest and after the cessation of exercise using a commercially available system (Predictor I, Arrhythmia Research Technology, Fitchburg, MA). All ECG recordings were made in the seated position. The rest recordings were made after a 5-min conditioning period. The ECG was sampled at a rate of 1,000 Hz. QRS complexes were detected using a template matching algorithm, and RR intervals were computed using custom software designed in Matlab (Mathworks, Natick, MA). The RR intervals preceding and the two RR intervals following the ectopic beats were excluded from the analysis.
HRV analysis.
Root mean square of successive differences of RR intervals (rMSSD) and root mean square residuals of linearly detrended RR intervals (RMS), two time-domain measures of HRV, were calculated for five consecutive 1-min segments. These measures have been previously validated to reflect parasympathetic reactivation during recovery immediately after exercise (14). RMS is equivalent to the SD of NN intervals after a linear detrending of the NN intervals. We hypothesized that rMSSD would more closely reflect HF HRV and that RMS would more closely reflect the total HRV power.
To obtain the frequency-domain measures of HRV, each of the five continuous 1-min segments of RR intervals were resampled at 4 Hz and then linearly detrended. After a Hanning window was applied, the power spectrum was then calculated for each segment using the fast Fourier transform on the 240 samples (4 Hz × 60 s) with a frequency resolution of 0.017 Hz. LF power was measured in the 0.04–0.15-Hz band (35a). HF power was measured in the 0.15- to 0.5-Hz band (35a). Total power (TOTAL) was calculated in the frequencies between 0.017- and 0.5-Hz. LF, HF, and TOTAL were analyzed as the square root of the power (sqrtLF, sqrtHF, and sqrtTOTAL) with units in milliseconds (rather than ms2 for power) so that these frequency-domain measures could be directly compared with the time-domain measures RMS and rMSSD using linear correlation. By Parseval's theorem, the total power calculated in the frequency domain is equivalent to the variance of the signal calculated in the time domain. Similarly, the sqrtTOTAL in the frequency domain corresponds to the SD of HRV in the time domain. LF-to-HF ratio was also calculated. Very LF power (0.003 to 0.04-Hz band) was not reported due to the lack of frequency resolution that is available in 1-min segments of NN intervals.
Respiratory rate estimation.
The HF power of HRV is known to be highly reflective of respiratory modulation of RR intervals (32). Therefore, an estimated respiratory signal was derived from the peak-to-peak amplitude of the QRS complexes. The respiratory rate was then computed by first resampling the QRS amplitude signal at 4 Hz and then obtaining the power spectrum using the fast Fourier transform. The dominant frequency between the 0.1- and 0.5-Hz band represents the respiratory rate in breaths per second.
Statistical analysis.
Time-dependent changes and the effect of parasympathetic and β-blockade on each of the HRV measures and respiratory rate were assessed using repeated-measures ANOVA. P values < 0.05 were considered statistically significant. Differences were identified with post hoc testing using the Holm-Sidak t-test for multiple comparisons. Correlation coefficients between the time- and frequency-domain HRV measures were computed.
RESULTS
RR intervals.
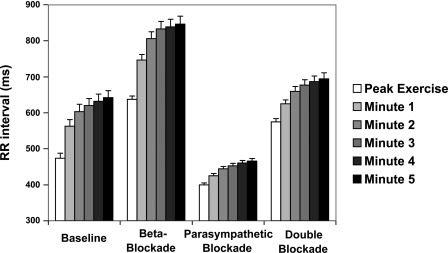
At rest, the subjects had mean RR intervals of 844 ± 107 ms. The RR intervals every minute starting at peak exercise for each of the four conditions of autonomic blockade are shown in Fig. 1. Over the 5 min of recovery, RR intervals obtained with β-adrenergic blockade were significantly longer than those obtained at baseline (784 ± 18 vs. 589 ± 18 ms, P < 0.0001), significantly shorter with parasympathetic blockade (441 ± 6 vs. 589 ± 18 ms, P < 0.0001), and significantly longer with double blockade (653 ± 13 vs. 589 ± 18 ms, P < 0.0001). RR intervals were significantly increased at the fifth minute compared with those at peak exercise for each of the four conditions (from 473 ± 14 to 642 ± 20 ms for baseline, from 637 ± 9 to 846 ± 22 ms with β-adrenergic blockade, from 444 ± 7 to 466 ± 7 ms with parasympathetic blockade, and from 575 ± 9 to 695 ± 17 ms with double blockade).
Fig. 1.
RR intervals (means ± SE) for peak exercise and the first 5 min of recovery after exercise for 4 conditions of autonomic blockade.
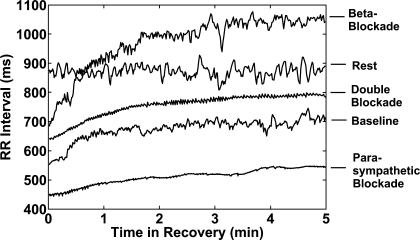
Figure 2 shows RR interval plots for the 5-min time period during rest and the first 5 min of recovery from exercise for each condition in a single subject. The RR intervals during rest show a high level of sustained variability. The RR intervals during recovery under baseline conditions and β-adrenergic blockade exhibited increasing HRV with time that approached resting levels at 5 min. The HRVs during parasympathetic blockade and double autonomic blockade are highly attenuated compared with those during baseline and β-adrenergic blockade and do not appear to significantly increase with time.
Fig. 2.
RR interval plots during preexercise rest and for the first 5 min of recovery after exercise. Significant heart rate variability is seen in the preexercise resting RR intervals. The recovery RR intervals at baseline and with β-blockade show low variability that increases with time. The RR intervals with parasympathetic blockade and double blockade have low variability throughout the 5 min of recovery.
Blood pressure.
Blood pressure measurements were taken at rest, at peak exercise, and at 5 min of recovery for each of the four conditions of autonomic blockade. Rest, peak exercise, and 5-min recovery systolic blood pressures were 120 ± 3, 155 ± 5, and 128 ± 4 mmHg for baseline, respectively; 113 ± 3, 134 ± 4, and 116 ± 4 mmHg for β-adrenergic blockade, respectively; 120 ± 4, 151 ± 4, and 123 ± 5 mmHg for parasympathetic blockade, respectively; and 115 ± 4, 133 ± 4, and 108 ± 3 mmHg for double blockade, respectively. Blood pressures at peak exercise and 5 min recovery were significantly less with β-adrenergic blockade (P < 0.0002) and with double blockade (P < 0.005) than at baseline but not significantly different with parasympathetic blockade. Rest, peak exercise, and 5-min-recovery diastolic blood pressures were 77 ± 2, 81 ± 2, and 75 ± 2 mmHg for baseline, respectively; 72 ± 2, 81 ± 2, and 72 ± 2 mmHg for β-adrenergic blockade, respectively; 72 ± 3, 79 ± 2, and 75 ± 2 mmHg for parasympathetic blockade, respectively; and 72 ± 2, 76 ± 2, and 71 ± 2 mmHg for double blockade, respectively. Blood pressures at peak exercise and 5 min recovery were less with double blockade than at baseline (P < 0.04).
Resting HRV.
At rest, the subjects had mean RR intervals of 844 ± 107 ms. The HRV measures during resting conditions are shown in Table 1.
Table 1.
Resting HRV values
| Parameter | Resting Values |
|---|---|
| RMS, ms | 26±13 |
| rMSSD, ms | 21±12 |
| LF, ms2 | 142±56 |
| HF, ms2 | 54±14 |
| TOTAL, ms2 | 299±79 |
| sqrtLF, ms | 10.0±1.2 |
| sqrtHF, ms | 6.1±0.8 |
| sqrtTOTAL, ms | 15.4±1.5 |
| LF/HF | 6.2±1.5 |
Values are means ± SE. HRV, heart rate variability; RMS, root mean square residuals of linearly detrended RR intervals; rMSSD, root mean square of successive differences of RR intervals; sqrtLF, square root of low-frequency power (LF); sqrtHF, square root of high-frequency power (HF); sqrtTOTAL, square root of total power (TOTAL).
Respiration.
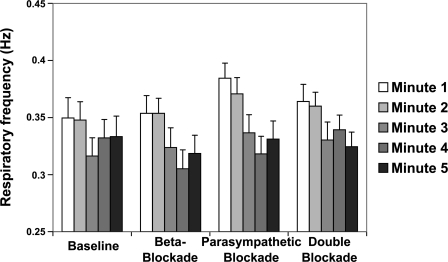
At rest, the subjects had an average respiratory rate of 0.28 ± 0.01 Hz (17 ± 3 breaths/min) as obtained from the estimated respiratory signal. Figure 3 shows the respiratory rate for the 5 min of recovery from exercise for each of the four conditions of autonomic blockade. Respiratory rate is increased at end exercise compared with rest (from 0.28 ± 0.01 to 0.36 ± 0.01 Hz, P < 0.0001) and decreases significantly during the first 5 min of recovery from exercise (from 0.36 ± 0.01 to 0.33 ± 0.01 Hz, P < 0.001). There were no significant differences in the respiratory rates with any of the four conditions of autonomic blockade.
Fig. 3.
ECG-derived respiratory frequency (means ± SE) plotted vs. time during recovery after exercise. The respiratory frequencies decreased with time. Selective autonomic blockade had no effect on the respiratory frequencies.
Effect of autonomic blockade on HRV during recovery.
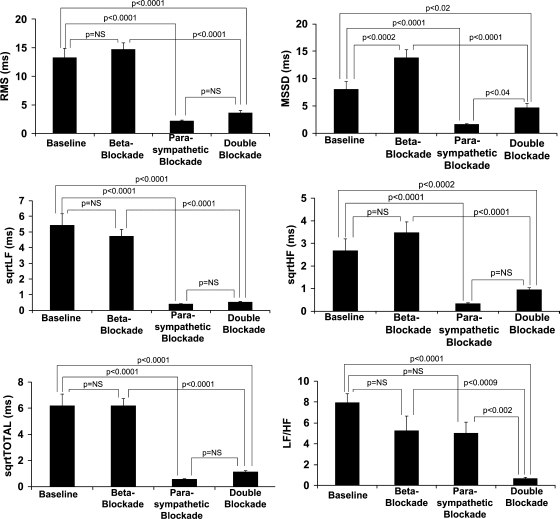
The effects of β-adrenergic blockade, parasympathetic blockade, and double blockade on HRV are summarized in Fig. 4. The means of the five 1-min segments for each condition are presented. The time-domain measure RMS demonstrated a significant decrease from baseline with parasympathetic blockade (P < 0.0001) and double blockade (P < 0.0001) but no difference with β-adrenergic blockade. rMSSD was also significantly decreased by parasympathetic blockade (P < 0.0001) and double blockade (P < 0.02), but increased by β-adrenergic blockade (P < 0.0002).
Fig. 4.
Bar graphs of the time- and frequency-domain measures of heart rate variability (means ± SE) for the 4 conditions of autonomic blockade averaged over the first 5 min of recovery from exercise. rMSSD, root mean square of successive differences of RR intervals; RMS, root mean square residuals of linearly detrended RR intervals; sqrtLF, square root of low-frequency power; sqrtHF, square root of high-frequency power; sqrtTOTAL, square root of total power; NS, not significant.
As shown in Fig. 4, the frequency-domain measures sqrtLF, sqrtHF, and sqrtTOTAL showed similar trends with autonomic blockade. The measures were significantly decreased with parasympathetic blockade and double blockade but showed no significant differences with β-adrenergic blockade compared with baseline. The measures with double blockade were not significantly different from those with parasympathetic blockade alone. The results for LF-to-HF ratio are also shown in Fig. 4. The LF-to-HF ratio was significantly decreased only by double blockade (P < 0.0001). β-Adrenergic and parasympathetic blockade did not significantly alter the LF-to-HF ratio compared with baseline. The LF-to-HF ratio with double blockade was, however, significantly less than that with β-adrenergic (P < 0.0009) or parasympathetic blockade (P < 0.002) alone.
Effect of recovery time after exercise on HRV.
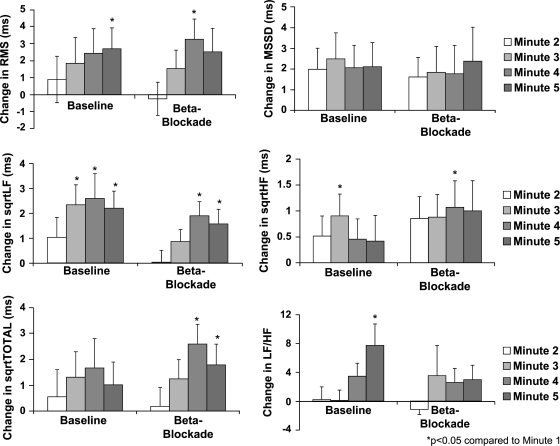
The first 5 min of recovery is characterized by the shifting of sympathovagal control toward increasing parasympathetic and decreasing sympathetic effect. To examine the response of the HRV measures to this shifting of sympathovagal control, the second through fifth minutes of recovery were compared with the first minute of recovery. Because of the low HRV values with parasympathetic blockade and double blockade, only the baseline and β-adrenergic blockade conditions were analyzed. A subset of these results is shown in Fig. 5.
Fig. 5.
Bar graphs of the change in heart rate variability for the second, third, fourth, and fifth minutes of recovery after exercise relative to the first minute of recovery.
As shown in Fig. 5, RMS showed a significant increase over the first minute for the baseline study by the fifth minute and a significant increase by the fourth minute with β-adrenergic blockade. rMSSD had an increasing trend after the first minute for baseline and with β-adrenergic blockade, but it was not statistically significant.
SqrtLF showed a sustained significant increase over the first minute by the third minute for the baseline study and by the fourth minute with β-adrenergic blockade. SqrtHF showed a significant increase at the third minute of the baseline study and the fourth minute of β-adrenergic blockade. SqrtTOTAL did not show significant changes at baseline but had changes in the fourth and fifth minute with β-adrenergic blockade. The LF-to-HF ratio showed a significant increase at the fifth minute of recovery, but significant increases were not detected at any time with β-adrenergic blockade.
Summary of HRV response to autonomic blockade and recovery time.
Multiple patterns of response were noted among the HRV parameters over time and with selective blockade. Table 2 provides a summary of the qualitative changes in each parameter relative to the expected autonomic changes in recovery. The specific factors include whether a significant change in the parameter was observed with β-adrenergic or parasympathetic blockade with arrows indicating the direction, whether the effects of β-adrenergic and parasympathetic blockade were in the opposite direction, and whether the parameter changed over the recovery time period consistent with the selective blockade with an arrow indicating direction. From Table 2, only the RR intervals met all five conditions, which suggests that the RR interval can reflect both steady-state and dynamic changes in sympathovagal influence.
Table 2.
Summary of RR interval and HRV response to autonomic blockade and recovery time
| β-Blockade Effect | Parasympathetic Blockade Effect | Opposite β-Blockade and Parasympathetic Blockade Effects | Time Effect | Time Effect Consistent with Blockade | |
|---|---|---|---|---|---|
| RR | Yes ↑ | Yes ↓ | Yes | Yes ↑ | Yes |
| RMS | No | Yes ↓ | N/A | Yes ↑ | Yes |
| rMSSD | Yes ↑ | Yes ↓ | Yes | No | N/A |
| sqrtLF | No | Yes ↓ | N/A | Yes ↑ | Yes |
| sqrtHF | No | Yes ↓ | N/A | Yes ↑ | Yes |
| sqrtTOTAL | No | Yes ↓ | N/A | Yes ↑ | Yes |
| LF/HF | No | No | N/A | Yes ↑ | N/A |
For the measures that were positively affected by autonomic blockade or time, the up arrows signify an increase in the measure and the down arrows signify a decrease. N/A, not applicable.
Correlation between time- and frequency-domain HRV measures.
Correlation coefficients were calculated between the time- and frequency-domain measures of HRV for the baseline conditions (shown in Table 3). RMS was most closely correlated with sqrtTOTAL with a correlation coefficient of 0.96, and rMSSD was most closely correlated with sqrtHF with a correlation coefficient of 0.95.
Table 3.
Correlation coefficients between frequency-domain and time-domain HRV measures
| RMS |
rMSSD |
|||
|---|---|---|---|---|
| r | P value | r | P value | |
| sqrtLF | 0.91 | <0.0001 | 0.78 | <0.0001 |
| sqrtHF | 0.86 | <0.0001 | 0.95 | <0.0001 |
| sqrtTOTAL | 0.96 | <0.0001 | 0.83 | <0.0001 |
DISCUSSION
The present study shows the effect of the change in sympathovagal control that occurs during recovery from exercise on time- and frequency-domain measures of HRV. The application of selective autonomic blockade near end exercise allowed for the evaluation of the autonomic effects on HRV during recovery. This demonstrated that an analysis of the commonly defined frequency bands of HRV spectra responds in predictable ways. Although there is reduction in total power (total HRV), HF continues to represent the respiratory modulation of parasympathetic input to the sinus node. Similarly, LF appears to be affected by both sympathetic and parasympathetic inputs. However, these frequency-domain measures do not provide any obvious benefit over using the time-domain measures such as RMS and rMSSD. Thus the study of sympathovagal changes during recovery from exercise might be performed using time-domain measures without the computational cost of RR interval resampling and fast Fourier transformation. This may be particularly important in the context of HRV monitoring in real-time or ambulatory settings.
HRV has been considered to be a marker of sympathetic and parasympathetic modulation of the heart rate in which both time- and frequency-domain analyses have been used as measures. Since the validation of these methods has been performed during steady-state heart rates, it is unclear whether these techniques can be applied to the condition of changing heart rates, such as the setting of exercise and postexercise recovery period. Furthermore, HRV has been shown to be attenuated during exercise in both HF and LF bands and the respiratory frequency is increased (2, 5, 33). Thus the evaluation of the physiological correlates of the LF and HF power is necessary.
Although LF power, when normalized by HF power or total power, had been previously identified as an index of sympathovagal influence, the results from using these indexes have been inconsistent in both the setting of exercise and recovery from exercise. No previous study had validated the physiological relationships of these parameters to autonomic effects using selective autonomic blockade during recovery from exercise.
Although the term “sympathovagal control” is not always easy to characterize in the setting of recovery from exercise, it has a clear definition as the sympathovagal control shifts to more parasympathetic effect and less sympathetic effect. In order for the HRV measures analyzed in this study to reflect this change in sympathovagal control, the following should be noted in the setting of recovery after exercise: 1) parasympathetic blockade and β-adrenergic blockade should have opposite effects on the HRV measures and 2) the HRV measure should change with time during recovery, consistent with the direction in which β-adrenergic blockade changes the HRV measure and opposite to the direction in which parasympathetic blockade changes the HRV measure. The mean RR intervals, as shown in Table 2, fulfilled each of these requirements. RR intervals were increased by β-adrenergic blockade but decreased by parasympathetic blockade. RR intervals increased with time for all four conditions of autonomic blockade. The direction of change with time was the same as the direction of change with β-adrenergic blockade and opposite of that of parasympathetic blockade. Thus RR intervals effectively reflected sympathovagal control, as has been previously demonstrated (14a).
Of the HRV measures, only rMSSD showed significant and opposite effects with β-adrenergic and parasympathetic blockade. However, significant time-dependent changes were not detected. RMS, sqrtLF, sqrtHF, and sqrtTOTAL showed changes with parasympathetic blockade but not β-adrenergic blockade. The time-dependent changes of these four measures were opposite in direction to the changes with parasympathetic blockade, suggesting that they reflect a parasympathetic reactivation following exercise. SqrtTOTAL was the most sensitive to the changes in sympathovagal control with time. Autonomic blockade did not produce the expected changes for LF-to-HF ratio. The LF-to-HF ratio changed with time but in the unexpected direction.
One of the implications of this study is that frequency-domain analysis of HRV, although validated in the situation of tilt table testing (27), may not be optimally applied in all situations as shown in the results during recovery from exercise. None of the frequency-domain measures either alone or in combination could differentiate β-adrenergic withdrawal from parasympathetic reactivation during the recovery from exercise. It is unknown whether these findings were complicated by the rapid heart rate changes or whether HRV has a different frequency-domain profile during recovery from exercise. There is a marked reduction of HRV that occurs during exercise and parasympathetic blockade that can prevent meaningful analysis in the frequency domain in these situations. However, there is clearly an analyzable amount of HRV that returns during the recovery of exercise (e.g., compare rest and baseline in Fig. 2). The ECG derived respiratory signals suggest that the defined frequency band for HF was adequate for the detection of respiratory modulation of RR intervals.
A second implication of this study is that the time-domain measures, RMS and rMSSD, are good surrogates for sqrtTOTAL and sqrtHF, respectively. The RMS measure we previously proposed (14) is a similar measurement to SD that can be used for nonstationary heart rates, such as in the setting of recovery immediately following exercise. However, instead of quantifying the fluctuation around the mean, RMS quantifies the fluctuation around the linear trend line. Because RMS measures the deviation from the linear trend line, RMS is sensitive to both LF and HF fluctuations. rMSSD, on the other hand, quantifies sample-to-sample changes and therefore is more sensitive to HF fluctuations of RR intervals than LF fluctuations. Both these measures have been previously shown to reflect a parasympathetic effect (14) and in this study were shown to reflect β-adrenergic effect as well. The high correlation of RMS with sqrtTOTAL and rMSSD with sqrtHF suggests that time-domain measures of HRV may be adequate in describing the LF and HF fluctuations of HRV during the postexercise recovery period without the computational cost of performing frequency-domain analysis.
In addition to the reduced computational cost, the time-domain analysis may have other potential advantages over frequency-domain analysis. Time-domain HRV measures avoid the use of empirically determined cutoffs for the HF and LF bands, removes the concern of the lack of frequency resolution when analyzing shorter segments of data, and avoids other anomalies that may occur when analyzing waveforms with multiple and changing frequencies in the frequency domain.
Limitations.
The autonomic effects studied were limited to recovery and not exercise. The autonomic milieu in the recovery period is complex as it changes over time, and there are nonlinear interactions between the sympathetic and parasympathetic nervous system effects in the control of heart rate with the sensitivity of one component being dependent on the level of activity of the other (13, 19, 34). Because of the integrated responses, the quantitative analysis using selective autonomic blockade may be inaccurate. However, we believe that the qualitative analysis is still useful. The time-dependent analysis of HRV required shorter durations of analysis than is typically used for frequency-domain measures of HRV. Thus the frequency resolution was not sufficient to calculate very LF. Although baroreceptive mechanisms are some of the major contributor of LF oscillations, measurements of arterial blood pressure were not performed throughout this study. Finally, it is not known whether the recovery results of this study can be generalized to exercise protocols different than the one used in this study. Lucini et al. (22) and Iellamo et al. (16) showed expected changes in LF and HF during exercise when using submaximal exercise protocols.
Conclusions.
In this study, frequency-domain analysis of HRV in the setting of recovery after exercise was tested for the first time using autonomic blockade. The LF and HF bands of the HRV power spectrum showed the expected response to parasympathetic and sympathetic blockade. Importantly, the time-domain measures RMS and rMSSD were shown to effectively reflect the autonomic effects in recovery and therefore have the potential for more widespread applicability.
Since autonomic changes in recovery, as measured by the 1-min heart rate recovery, have been shown to have important prognostic implications (3, 4, 7, 8, 10–12, 20, 36), the current data provide the physiological framework to further study the prognostic significance of HRV in the postexercise recovery period.
GRANTS
This research was supported in part by National Institutes of Health Grants M01-RR-00048 (to the General Clinical Research Center of Northwestern Memorial Hospital) and 1-RO1-HL-70179-01A2.
REFERENCES
- 1.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 213: 220–222, 1981 [DOI] [PubMed] [Google Scholar]
- 2.Arai Y, Saul JP, Albrecht P, Hartley LH, Lilly LS, Cohen RJ, Colucci WS. Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol Heart Circ Physiol 256: H132–H141, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Bernardi L, Salvucci F, Suardi R, Solda PL, Calciati A, Perlini S, Falcone C, Ricciardi L. Evidence for an intrinsic mechanism regulating heart rate variability in the transplanted and the intact heart during submaximal dynamic exercise? Cardiovasc Res 24: 969–981, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 85: 164–171, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Breuer HW, Skyschally A, Schulz R, Martin C, Wehr M, Heusch G. Heart rate variability and circulating catecholamine concentrations during steady state exercise in healthy volunteers. Br Heart J 70: 144–149, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadei B, Cochrane S, Johnston J, Conway J, Sleight P. Pitfalls in the interpretation of spectral analysis of the heart rate variability during exercise in humans. Acta Physiol Scand 153: 125–131, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 341: 1351–1357, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Cripps TR, Malik M, Farrell TG, Camm AJ. Prognostic value of reduced heart rate variability after myocardial infarction: clinical evaluation of a new analysis method. Br Heart J 65: 14–19, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrell TG, Bashir Y, Cripps T, Malik M, Poloniecki J, Bennett ED, Ward DE, Camm AJ. Risk stratification for arrhythmic events in postinfarction patients based on heart rate variability, ambulatory electrocardiographic variables and the signal-averaged electrocardiogram. J Am Coll Cardiol 18: 687–697, 1991 [DOI] [PubMed] [Google Scholar]
- 11.Fauchier L, Babuty D, Cosnay P, Autret ML, Fauchier JP. Heart rate variability in idiopathic dilated cardiomyopathy: characteristics and prognostic value. J Am Coll Cardiol 30: 1009–1014, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Fukuta H, Hayano J, Ishihara S, Sakata S, Mukai S, Ohte N, Ojika K, Yagi K, Matsumoto H, Sohmiya S, Kimura G. Prognostic value of heart rate variability in patients with end-stage renal disease on chronic haemodialysis. Nephrol Dial Transplant 18: 318–325, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Furukawa Y, Hoyano Y, Chiba S. Parasympathetic inhibition of sympathetic effects on sinus rate in anesthetized dogs. Am J Physiol Heart Circ Physiol 271: H44–H50, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Goldberger JJ, Le FK, Lahiri M, Kannankeril PJ, Ng J, Kadish AH. Assessment of parasympathetic reactivation after exercise. Am J Physiol Heart Circ Physiol 290: H2446–H2452, 2006 [DOI] [PubMed] [Google Scholar]
- 14a.Goldberger JJ. Sympathovagal balance: how should we measure it? Am J Physiol Heart Circ Physiol 276: H1273–H1280, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Iellamo F. Neural mechanisms of cardiovascular regulation during exercise. Auton Neurosc 90: 66–75, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Iellamo F, Pizzinelli P, Massaro M, Raimondi G, Peruzzi G, Legramante JM. Muscle metaboreflex contribution to sinus node regulation during static exercise: insights from spectral analysis of heart rate variability. Circulation 100: 27–32, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, Takeda H, Inoue M, Kamada T. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol 24: 1529–1535, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Jose AD, Collison D. The normal range and determinants of the intrinsic heart rate in man. Cardiovasc Res 4: 160–167, 1970 [DOI] [PubMed] [Google Scholar]
- 19.Kawada T, Sugimachi M, Shishido T, Miyano H, Ikeda Y, Yoshimura R, Sato T, Takaki H, Alexander J, Jr, Sunagawa K. Dynamic vagosympathetic interaction augments heart rate response irrespective of stimulation patterns. Am J Physiol Heart Circ Physiol 272: H2180–H2187, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 59: 256–262, 1987 [DOI] [PubMed] [Google Scholar]
- 21.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 351: 478–484, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Lucini D, Trabucchi V, Malliani A, Pagani M. Analysis of initial autonomic adjustments to moderate exercise in humans. J Hypertens 13: 1660–1663, 1995 [PubMed] [Google Scholar]
- 23.Maciel BC, Gallo L, Jr, Marin Neto JA, Lima Filho EC, Martins LE. Autonomic nervous control of the heart rate during dynamic exercise in normal man. Clin Sci (Lond) 71: 457–460, 1986 [DOI] [PubMed] [Google Scholar]
- 24.Martinmaki K, Rusko H. Time-frequency analysis of heart rate variability during immediate recovery from low and high intensity exercise. Eur J Appl Physiol 102: 353–360, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Nakamura Y, Yamamoto Y, Muraoka I. Autonomic control of heart rate during physical exercise and fractal dimension of heart rate variability. J Appl Physiol 74: 875–881, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Oida E, Moritani T, Yamori Y. Tone-entropy analysis on cardiac recovery after dynamic exercise. J Appl Physiol 82: 1794–1801, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell'Orto S, Piccaluga E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 59: 178–193, 1986 [DOI] [PubMed] [Google Scholar]
- 28.Parekh A, Lee CM. Heart rate variability after isocaloric exercise bouts of different intensities. Med Sci Sports Exerc 37: 599–605, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Perini R, Orizio C, Baselli G, Cerutti S, Veicsteinas A. The influence of exercise intensity on the power spectrum of heart rate variability. Eur J Appl Physiol Occup Physiol 61: 143–148, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Perini R, Orizio C, Comande A, Castellano M, Beschi M, Veicsteinas A. Plasma norepinephrine and heart rate dynamics during recovery from submaximal exercise in man. Eur J Appl Physiol Occup Physiol 58: 879–883, 1989 [DOI] [PubMed] [Google Scholar]
- 31.Pichon AP, de Bisschop C, Rouland A, Papelier Y. Spectral analysis of heart rate variability during exercise in trained subjects. Med Sci Sports Exerc 36: 1702–1708, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Sayers BM. Analysis of heart rate variability. Ergonomics 16: 17–32, 1973 [DOI] [PubMed] [Google Scholar]
- 33.Shin K, Minamitani H, Onishi S, Yamazaki H, Lee M. The power spectral analysis of heart rate variability in athletes during dynamic exercise—Part II. Clin Cardiol 18: 664–668, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Stramba-Badiale M, Vanoli E, De Ferrari GM, Cerati D, Foreman RD, Schwartz PJ. Sympathetic-parasympathetic interaction and accentuated antagonism in conscious dogs. Am J Physiol Heart Circ Physiol 260: H335–H340, 1991 [DOI] [PubMed] [Google Scholar]
- 35.Sundaram S, Carnethon M, Polito K, Kadish AH, Goldberger JJ. Autonomic effects on QT-RR interval dynamics after exercise. Am J Physiol Heart Circ Physiol 294: H490–H497, 2008 [DOI] [PubMed] [Google Scholar]
- 35a.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93: 1043–1065, 1996 [PubMed] [Google Scholar]
- 36.Tsuji H, Venditti FJ, Jr, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation 90: 878–883, 1994 [DOI] [PubMed] [Google Scholar]
- 37.Warren JH, Jaffe RS, Wraa CE, Stebbins CL. Effect of autonomic blockade on power spectrum of heart rate variability during exercise. Am J Physiol Regul Integr Comp Physiol 273: R495–R502, 1997 [DOI] [PubMed] [Google Scholar]