Abstract
The mirror neuron system (MNS) is a trimodal system composed of neuronal populations that respond to motor, visual, and auditory stimulation, such as when an action is performed, observed, heard or read about. In humans, the MNS has been identified using neuro-imaging techniques (such as fMRI and mu suppression in the EEG). It reflects an integration of motor-auditory-visual information processing related to aspects of language learning including action understanding and recognition. Such integration may also form the basis for language-related constructs such as theory of mind. In this article, we review the MNS system as it relates to the cognitive development of language in typically developing children and in children at-risk for communication disorders, such as children with autism spectrum disorder (ASD) or hearing impairment. Studying MNS development in these children may help illuminate an important role of the MNS in children with communication disorders. Studies with deaf children are especially important because they offer potential insights into how the MNS is reorganized when one modality, such as audition, is deprived during early cognitive development, and this may have long-term consequences on language maturation and theory of mind abilities.
Learning outcomes
Readers will be able to (1) understand the concept of mirror neurons, (2) identify cortical areas associated with the MNS in animal and human studies, (3) discuss the use of mu suppression in the EEG for measuring the MNS in humans, and (4) discuss MNS dysfunction in children with (ASD).
Keywords: Mirror neurons, Autism, EEG, Mu rhythm, Children
1. Introduction
The mirror neuron system (MNS) is a recently discovered, trimodal system for action understanding and recognition that responds to auditory, visual, and motor stimulation (D'Ausilio, 2007). ‘Mirror neurons’ have been directly observed in primate studies using depth electrodes (Gallese, Keysers, & Rizzolatti, 2004; Rizzolatti, Fadiga, Gallese, & Fogassi, 1996; Rizzolatti, Fogassi, & Gallese, 2001) and localized to area F5, which is thought to be a homolog of Broca's area, to the superior temporal sulcus (STSa), a possible homolog Wernicke's area, and the inferior parietal cortex (Rizzolatti et al., 2001; Rizzolatti & Arbib, 1998). These neurons have been shown to discharge both when a monkey performs specific hand actions, such as grasping an object, and when it observes the same action performed by another monkey or human (Gallese, Fadiga, Fogassi, & Rizzolatti, 1996; Rizzolatti et al., 1996, 2001). That is, the neurons ‘mirror’ the behavior of another individual. Furthermore, area F5 contains audio-visual mirror neurons that discharge not only when an action is observed or performed, but also when only the sound of that action is heard (Kohler et al., 2002). Hence, it has been hypothesized that the MNS responds to the execution of a movement as well as the audiovisual representations of the same movement, providing an audiovisual-to-motor link or integration between seeing, hearing and doing (Pineda, 2005). In children where the MNS is dysfunctional this audiovisual-to-motor link will be deficient and potentially result in a number of problems, including communication disorders.
Recent studies have inferred the presence of mirror neurons in humans using neuroimaging methods. For example, functional magnetic resonance imaging (fMRI) studies have shown that neurons within the ventral premotor cortex, centered in Broca's area, respond during the observation of arm and hand actions suggesting human correlates of mirror neurons described in the animal studies (Buccino et al., 2001). Gazzola, Aziz-Zadeh, & Keysers (2006) have reported activation of the left hemispheric temporo-parieto-premotor circuit in humans, an area that responds both during the motor execution of an action and when individuals listen to the sound of that action (examples include ripping paper and breaking peanuts). Similar neuroimaging studies have also revealed areas in the human premotor cortex that respond both when participants observe actions being performed and when they read phrases relating to those actions, such as “biting the banana” or “grasping the pen” (Aziz-Zadeh, Wilson, Rizzolatti, & Iacoboni, 2006b). A number of additional regions of the human cortex appear to have mirror properties, including the superior temporal sulcus (STS) and the extrastriate body area, suggesting a broader mirror neuron system than is typically described (Pineda, 2008). Nonetheless, the animal and human studies, taken together, suggest that an action-observation-execution matching system similar to that reported in the primate studies, also exists in humans (see Oberman & Ramachandran 2007 for a detailed review of human neuro-anatomical substrates of the mirror neuron system).
While the primate and human MNS share the basic properties of action execution-observation, the human MNS appears to extend its function to other aspects of social cognition, more specifically to language and communication as well as imitation learning (Oberman et al., 2005; Oberman, Pineda, & Ramachandran, 2006; Oberman, Ramachandran, & Pineda, 2008). Indeed, the discovery of mirror neurons in monkeys and a homologous “mirror system” in Broca's area in the human brain has revived the ‘gestural origins’ theory of the evolution of the human capability for language (Rizzolatti and Arbib, 1998; Arbib, 2005; 2008). This has encouraged the exploration of the role of the MNS in special populations such as autism spectrum disorder (ASD) and deaf children, since Broca's area contains representations for arm and other movements that are implicated in verbal-and sign-based language systems (Rizzolatti & Arbib, 1998; Rizzolatti et al., 2001).
2. MNS and cognitive development
Given its functional properties, the MNS appears to play a role in allowing children to learn from other people's actions and to generalize through observation and imitation, by mapping such actions onto their own actions and language. The ‘direct matching hypothesis’ put forward by Rizzolatti et al., 2001, suggests that we understand actions and intentions by mapping the auditory/visual representation of the observed action onto our motor representation of the same action. Thus, once the actions of another individual are represented and understood in terms of one's own actions, it is possible to predict the mental state of the observed individual, leading to a ‘theory of mind’ (Oberman et al., 2005, 2006, 2008; Oberman & Ramachandran, 2007; Pineda, 2005; Rizzolatti et al., 2001). Theory of mind refers to our ability to infer another person's mental state, including their beliefs and desires, from their experiences and behavior. Hence, if we watch a person reaching into a box labeled “crayons,” we can assume that he/she wants a crayon and that the individual believes there are crayons in the box (Schick, de Villiers, de Villiers, & Hoffmeister, 2007). Theory of mind thus relates to cognitive development and social communication because it provides a fundamental ability to understand the actions and intentions of others, and to communicate them effectively.
Listening to, and reading action-related words and sentences such as “to pick” or “he kicked the ball” have been shown to invoke mirror neuron activity implicating the MNS in language processing and comprehension (Buccino et al., 2005; Gazzola et al., 2006; Hauk & Pulvermuller, 2004; Tettamanti et al., 2005). TMS studies have provided further evidence for the modulation of motor areas involved in speech production when listening to action-related sentences (Buccino et al., 2005), suggesting a possible role for the MNS in speech comprehension (Oberman & Ramachandran, 2007). In general, action-related language may play a useful role in language development because it provides a capacity for children to not only observe, embody, and perform the actions, but to communicate the meaning and intentions behind such actions using a symbolic language.
As previously described, there is now substantial evidence for the existence of a human MNS, and some of its properties have been delineated with respect to imitation learning, social communication and language development. However, much more research is needed to fully comprehend how the MNS relates functionally to the neurobiological development of cognition and language, and whether deficits in MNS may underlie cognitive and/or language disorders. Thus, studies that address the development of the MNS in both typically developing children and in children with a communication disorder such as in ASD and hearing impaired is the next step in understanding the role of the MNS.
3. EEG correlates of the human MNS
In addition to fMRI studies, there is now electroencephalography (EEG) evidence for MNS activity in humans, including young children (Lepage & Theoret, 2006). Suppression of EEG oscillations in the mu frequency range (8-13Hz) over the sensorimotor cortex appears to be correlated with mirror neuron activity (Oberman et al., 2005, 2006). For example, suppression of EEG brain rhythms in the mu frequency range occurs when observing, performing, and hearing actions in individuals with a properly functioning MNS (Pineda, 2005). However, no such modulation is present when subjects watch object movements that are not performed by humans (Cochin, Barthelemy, Lejeune, Roux, & Martineau, 1998). Converging evidence from studies from many laboratories demonstrate that mu power is reduced (suppressed) in adults, both when they perform actions and when they observe others performing actions, reflecting the observation/execution system associated with the MNS (Oberman et al., 2005, 2006, 2008; Pineda, 2005).
In Fig. 1, we show mu suppression in EEG recordings from a group of 10 typical young adults (ages 19-27 years). Recordings are from electrodes along the coronal plane at the midline (Cz), left (C3) and right (C4) hemispheres along the coronal plane. Mu suppression was measured in conditions where the subject performed an action (opening and closing their hand), and when they observed another person performing the same action on a video screen, consistent with previous studies (Obermann et al., 2005). EEG evidence for the action observation-execution mechanism within the MNS has also recently been verified in children (Lepage & Theoret, 2006; Oberman et al., 2008). Given that EEG recordings are easily and routinely obtained in pediatric populations, EEG mu suppression provides a noninvasive and cost effective technique to examine development of the MNS in children who have communication disorders.
Fig. 1.
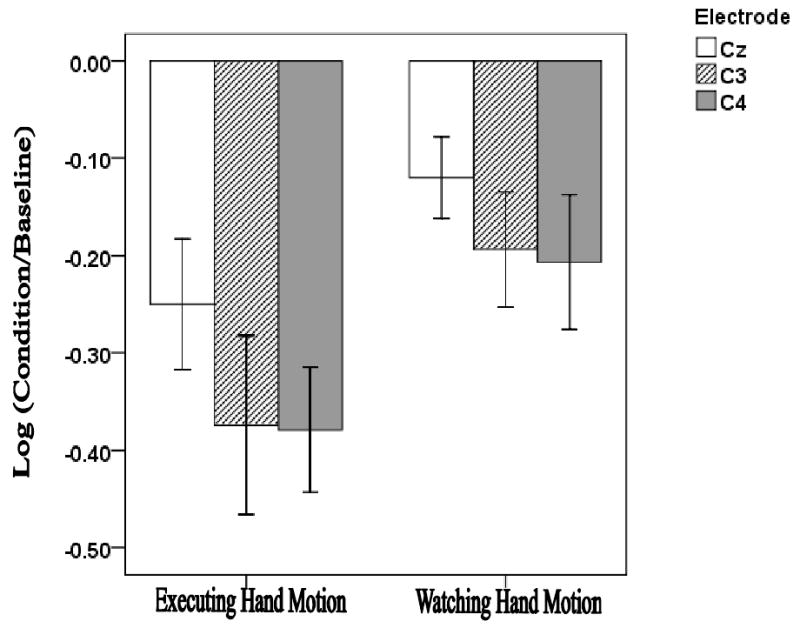
Mu suppression in EEG recordings from a group of 10 typical young adults (ages 19-27 years) is shown. Recordings are from electrodes along the coronal plane at the midline (Cz), left (C3) and right (C4) hemispheres along the coronal plane. Mu suppression was measured in conditions where the subject executed an action (opening and closing their hand), and when they observed another person performing the same action on a video screen.
Studies involving the MNS and language comprehension that have examined the modulation of the MNS while reading or listening to action-related words and sentences have been conducted only with TMS and fMRI neuroimaging techniques (Aziz-Zadeh et al., 2006b; Buccino et al., 2005; Gazzola et al., 2006; Tettamanti et al., 2005). Complimentary studies are needed that use mu suppression to examine MNS involvement in language development in typically developing children and in children with communication disorders. Our research group is currently pursuing studies with mu suppression and language that aim to illuminate developmental effects between younger and older children that correlate to language development and comprehension. Such studies may provide important insight into to the role of the MNS in cognitive disorders such as in children with ASD and deafness, which can address the impact of delayed language development and auditory deprivation on the MNS development.
4. MNS dysfunction in children with cognitive disorders
4.1 Children with autism
It is well established that children with ASD have deficits in imitation (Williams 2004; review). Because the MNS is implicated in imitation learning, recent studies have sought to examine the development of the MNS in children with ASD. Oberman et al. (2005) measured mu suppression in ten high-functioning individuals with ASD, and ten age- and gender-matched control subjects, while watching videos of (1) a moving hand, (2) a bouncing ball, and (3) virtual noise, and while also moving their own hand. Control subjects showed significant mu suppression to both self and observed hand movements, whereas the ASD group showed significant mu suppression only to self-performed hand movements and not to observed hand movements. In a follow-up study, Oberman et al. (2008) measured mu suppression in thirteen high-functioning children with ASD, and thirteen age- and gender-matched control subjects, all of whom were between the ages of 8 and 12 years. Mu suppression was measured while the children were watching videos of (1) a stranger opening and closing their right hand, (2) the child's guardian or sibling performing the same hand motion, (3) the participant's own hand performing the same action, and (4) a video of two bouncing balls moving vertically toward and away from each other. Results revealed that children with ASD (and typically developing children) showed greater mu suppression to actions performed by familiar individuals (such as their guardians/parents) compared to those of strangers. Taken together, the results from these two studies suggests that the MNS in individuals with ASD responds to observed actions, but only when the individuals can identify in some personal way with the stimuli, or the individuals engaged in the action (Oberman et al., 2008). These results are consistent with clinical observations of improvements in social skills and communication when ASD children interact with a parent or sibling, thus suggesting that family and familiarity may play an important role in therapy of individuals with ASD. Overall, results of these studies are consistent with other recent work that supports the role of an impaired MNS in individuals with ASD (Bernier, Dawson, Webb, & Murias, 2007; Dapretto et al., 2005; Theoret et al., 2005).
4.2 Children with cochlear implants
In typical development, the MNS is believed to integrate auditory, visual, and motor stimulation, therefore, it is likely that the development of this tri-modal system will be reorganized and perhaps deficient when one modality such as audition is deprived, for example in deaf children. It is possible that deficits in MNS development in deafness may underlie recently reported deficits in ‘theory of mind’ development in deaf children (Schick et al., 2007). Many of these children now receive cochlear implants at an early age, which allow normal development of central auditory pathways and auditory cortical areas (Sharma & Dorman, 2006). At present, studies in our laboratory are examining MNS development using mu suppression in children with cochlear implants. Of interest, will be whether cochlear implantation at an early age might prevent or alternately, overcome deficits in MNS development resulting in normal ‘theory of mind’ development in cochlear-implanted children.
5. Summary
While the functioning of the MNS in humans has not been completely delineated, it has been linked to aspects of cognitive development such as imitative learning, language acquisition and social communication. Recent studies have shown that children with cognitive disorders, such as children with ASD show deficits in MNS development. Because the MNS responds to the execution of a movement as well as the audiovisual representation of the same movement it is assumed to provide an audiovisual-to-motor integration between seeing, hearing and doing (Pineda, 2005). Such multimodal integration would seem to be critical for a number of social behaviors, including action comprehension and language development. Hence, problems with MNS in special populations, such as ASD and hearing-impaired children, may be the basis for communication disorders. Since MNS development can be studied using routine EEG techniques in children, future studies should examine MNS development in other pediatric populations who have, or are at-risk for developing communication disorders. It is our hope that such studies will address the important role of language and MNS development in children with communication disorders, which appears to be closely linked to theory of mind abilities that are necessary for social communication. Therefore, studying the motor-audio-visual processing of the MNS in deaf children will be particularly relevant.
Acknowledgments
Supported by grants from the Institute of Cognitive Science at University of Colorado to R.L and from the National Institutes of Health (NIH/NIDCD R01DC06257 and R01DC04552) to A.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arbib MA, editor. Action to language via the mirror neuron system. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- Arbib MA, Billard A, Iacoboni M, Oztop E. Synthetic brain imaging: Grasping, mirror neurons and imitation. Neural Networks. 2000;13(89):975–997. doi: 10.1016/s0893-6080(00)00070-8. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Koski L, Zaidel E, Mazziotta J, Iacoboni M. Lateralization of the human mirror neuron system. The Journal of Neuroscience. 2006a;26(11):2964–2970. doi: 10.1523/JNEUROSCI.2921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Wilson S, Rizzolatti G, Iacoboni M. Congruent embodied representations for visually presented actions and linguistic phrases describing actions. Current Biology. 2006b;16(18):1818–1823. doi: 10.1016/j.cub.2006.07.060. [DOI] [PubMed] [Google Scholar]
- Bernier R, Dawson G, Webb S, Murias M. EEG mu rhythm and imitation impairments in individuals with autism spectrum disorder. Brain and Cognition. 2007;64(3):228–237. doi: 10.1016/j.bandc.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccino G, Riggio L, Melli G, Binkofski F, Gallese V, Rizzolatti G. Listening to action-related sentences modulates the activity of the motor system: A combined TMS and behavioral study. Cognitive Brain Research. 2005;24(3):355–363. doi: 10.1016/j.cogbrainres.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, et al. Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. European Journal of Neuroscience. 2001;13:400–404. [PubMed] [Google Scholar]
- Cochin S, Barthelemy C, Lejeune B, Roux S, Martineau J. Perception of motion and qEEG activity in human adults. Electroencephalography and Clinical Neurophysiology. 1998;107(4):287–295. doi: 10.1016/s0013-4694(98)00071-6. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2005;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ausilio A. The role of the mirror system in mapping complex sounds into actions. The Journal of Neuroscience. 2007;27(22):5847–5848. doi: 10.1523/JNEUROSCI.0979-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Watkins KE. Stimulating language: Insights from TMS. Brain. 2007;130(3):610–622. doi: 10.1093/brain/awl331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: From action organization to intention understanding. Science. 2005;308(5722):662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends in Cognitive Sciences. 2004;8(9):396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Garbarini F, Adenzato M. At the root of embodied cognition: Cognitive science meets neurophysiology. Brain and Cognition. 2004;56:100–106. doi: 10.1016/j.bandc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Aziz-Zadeh L, Keysers C. Empathy and somatotopic auditory mirror system in humans. Current Biology. 2006;16(18):1824–1829. doi: 10.1016/j.cub.2006.07.072. [DOI] [PubMed] [Google Scholar]
- Hauk O, Pulvermuller F. Neurophysiological distinction of action words in the fronto-central cortex. Human Brain Mapping. 2004;21(3):191–201. doi: 10.1002/hbm.10157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one's own mirror neuron system. PLoS Biology. 2005;3(3):e79. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler E, Keysers C, Umiltà MA, Fogassi L, Gallese V, Rizzolatti G. Hearing sounds, understanding actions: Action representation in mirror neurons. Science. 2002;297(5582):846–848. doi: 10.1126/science.1070311. [DOI] [PubMed] [Google Scholar]
- Lepage JF, Theoret H. EEG evidence for the presence of an action observation-execution matching system in children. European Journal of Neuroscience. 2006;23:2505–2510. doi: 10.1111/j.1460-9568.2006.04769.x. [DOI] [PubMed] [Google Scholar]
- Luppino G, Rizzolatti G. The organization of the frontal motor cortex. News in Physiological Sciences. 2000;15(5):219–224. doi: 10.1152/physiologyonline.2000.15.5.219. [DOI] [PubMed] [Google Scholar]
- Nishitani N. Broca's region: From action to language. Physiology. 2005;20:60–69. doi: 10.1152/physiol.00043.2004. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Ramachandran VS, Pineda JA. Modulation of mu suppression in children with autism spectrum disorders in response to familiar or unfamiliar stimuli: The mirror neuron hypothesis. Neuropsychologia. 2008;46(5):1558–1565. doi: 10.1016/j.neuropsychologia.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Ramachandran VS. The simulating social mind: The role of the mirror neuron system and simulation in the social and communicative deficits of autism spectrum disorders. Psychological Bulletin. 2007;133(2):310–327. doi: 10.1037/0033-2909.133.2.310. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Pineda JA, Ramachandran VS. The human mirror neuron system: A link between action observation and social skills. Social Cognitive and Affective Neuroscience (SCAN) 2006;2(1):62–66. doi: 10.1093/scan/nsl022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cognitive Brain Research. 2005;24:190–198. doi: 10.1016/j.cogbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Pineda JA. Sensorimotor cortex as a critical component of an ‘extended’ mirror neuron system: Does it solve the development, correspondence, and control problems in mirroring? Behavioral and Brain Functions. 2008;4:47. doi: 10.1186/1744-9081-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda JA. The functional significance of mu rhythms: Translating “seeing” and “hearing” into “doing”. Brain Research Reviews. 2005;50(1):57–68. doi: 10.1016/j.brainresrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nature Reviews: Neuroscience. 2001;2(9):661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib MA. Language within our grasp. Trends in Neuroscience. 1998;21(5):188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Cognitive Brain Research. 1996;3(2):131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Schick B, de Villiers P, de Villiers J, Hoffmeister R. Language and theory of mind: A study of deaf children. Child Development. 2007;78(2):376–396. doi: 10.1111/j.1467-8624.2007.01004.x. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF. Central auditory development in children with cochlear implants: Clinical implications. In: Moller A, editor. Cochlear and brainstem implants, Advances in Otorhinolaryngology. Vol. 64. 2006. pp. 66–88. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Kral A. The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants. Hearing Research. 2005;203(12):134–143. doi: 10.1016/j.heares.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Buccino G, Saccuman MC, Gallese V, Danna M, Scifo P, et al. Listening to action-related sentences activates fronto-parietal motor circuits. Journal of Cognitive Neuroscience. 2005;17(2):273–281. doi: 10.1162/0898929053124965. [DOI] [PubMed] [Google Scholar]
- Theoret H, Halligan E, Kobayashi M, Fregni F, Tager-Flusber H, Pascual-Leone A. Impaired motor facilitation during action observation in individuals with autism spectrum disorder. Current Biology. 2005;15(3):84–85. doi: 10.1016/j.cub.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Williams JHG, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neuroscience and Behavioral Reviews. 2001;25:287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]


