Table 3.
In Vitro Activities of 3-Mono-, Di-, and Trifluoromethyl-7-substituted-THIQs.
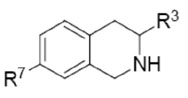 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R3= CH2F (a)a | R3= CHF2 (b)a | R3= CF3 (c)b | ||||||||
| Compd | R7 | PNMTc | α2c,d | Sele | PNMTc | α2c,d | Sele | PNMTc | α2c,d | Sele |
| 11a–c | Br | 0.023 | 6.4 | 280 | 0.094 | 230 | 2400 | 3.2 | >1000 | >310 |
| 12a–c | CF3 | 0.030 | 41 | 1400 | 0.067 | 190 | 2800 | 0.98 | >1000 | >1000 |
| 13a–c | I | 0.054 | 7.1 | 130 | 0.20 | >1000 | >5000 | 1.9 | >1000 | >530 |
| 14a–c | SO2NHCH2CF3 | 0.13 | 1200 | 9200 | 0.25 | >1000 | >4000 | 9.4 | >1000 | >110 |
| 15a–c | NO2 | 0.15 | 76 | 510 | 0.17 | >1000 | >5800 | 2.3 | 1400 | 610 |
| 16a–c | SO2NH2 | 0.15 | 680 | 4500 | 0.68 | >1000 | >1500 | 8.0 | >1000 | >125 |
| 17a–c | SO2NH(p-C6H4Cl) | 0.27 | 140 | 520 | 0.90 | >1000 | >1100 | 15 | >1000 | >67 |
| 18a–c | CN | 0.80 | 460 | 570 | 3.1 | >1000 | >320 | 21 | 2900 | 140 |
| 19a–c | H | 0.82 | 3.8 | 4.6 | 3.4 | 150 | 44 | 23 | 400 | 17 |
| 20a–c | SO2CH3 | 1.1 | 230 | 210 | 6.0 | 2500 | 420 | 41 | 3900 | 95 |
| 21a–c | SO2NHEt | 1.4 | 550 | 390 | 3.2 | >1000 | >310 | 60 | >1000 | >17 |
| 22a–c | SO2NHPr | 1.7 | 610 | 360 | 2.1 | >1000 | >480 | — | — | — |
| 23a–c | SO2NH(CH2)3OCH3 | 2.6 | 750 | 290 | 5.3 | >1000 | >190 | 13 | >1000 | >77 |
| 24a–c | SO2NHBu | 3.4 | 260 | 76 | 5.6 | >1000 | >180 | — | — | — |
| 25a–c | SO2NH(p-C6H4NO2) | 7.7 | >1000 | >130 | 37 | >1000 | >27 | 110 | >1000 | >9.1 |
Standard error of the mean was not greater than 10%.
In vitro activities for the inhibition of [3H]clonidine binding to the α2-adrenoceptor.
Selectivity α2 Ki /hPNMT Ki.
