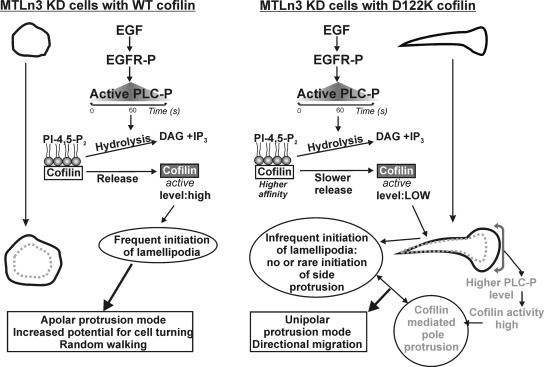
Figure 9.
PI(4,5)P2–cofilin interaction controls the mode of EGF-induced protrusion and migration in MTLn3 cells: model of underlying mechanisms in cofilin KD cells expressing either WT cofilin or D122K cofilin. The extent of release of active cofilin from PI(4,5)P2 within the timeframe of PLC activation and resulting PI(4,5)P2 hydrolysis is the key control point in lamellipodia initiation. Released cofilin is modeled to sever actin filaments in the periphery, an event followed by Arp2/3-mediated actin nucleation and lamellipodia extension. Uncompromised cofilin activation and lamellipodia initiation in WT cells results in protrusion along the entire periphery (apolar protrusion) and random walking. Due to its higher affinity for PI(4,5)P2, D122K is released slower resulting in insufficient release of cofilin and no new side protrusion formation. In the established lamellipodia in the pole, activated PLC is enriched (Chou et al., 2003; Mouneimne et al., 2006). This will locally result in stronger PI(4,5)P2 decrease, which we suggest compensates the slower D122K cofilin release and results in normal cofilin-mediated protrusion at the pole. In combination, this results in directional migration of cells expressing the mutant. EGFR-P, phosphorylated EGF receptor; PLC-P, phosphorylated (active) phospholipase C; DAG, diacylglycerol; and IP3, inositol-1,4,5-trisphosphate.