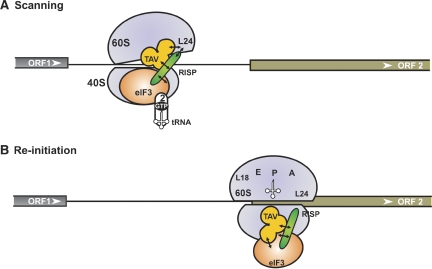
Figure 7.
Proposed model of re-initiation supporting protein (RISP) function in 60S recruitment during virus-activated re-initiation. We propose the following scenario: during ORF1 elongation, the RISP–transactivator viroplasmin (TAV)–80S complex can be stabilized by transfer of TAV–RISP–eIF3 to the solvent surface of 60S through TAV binding to L18/L13. During termination, the TAV–RISP–eIF3 complex is relocated back to 40S to reconstruct a pre-initiation complex (PIC) competent for re-initiation. (A) RISP–TAV establishes a bridge between 40S-bound eIF3 and 60S through the ribosomal protein L24, preventing, for a short time, removal of 60S. During scanning, RISP bridges the relaxed 40S–60S interactions through contact with 40S-bound eIF3, while simultaneously stabilizing TAV–L24 contacts (open conformation of 80S). This open 80S conformation allows eIF3-bound 40S to continue scanning and search for a downstream start codon. (B) Codon–anticodon recognition and positioning of Met-tRNAiMet in the ribosomal P-site would then displace TAV and RISP from L24 followed by the formation of 80S ready for elongation. eIF3, TC, RISP, TAV, L24, 40S and 60S are indicated.