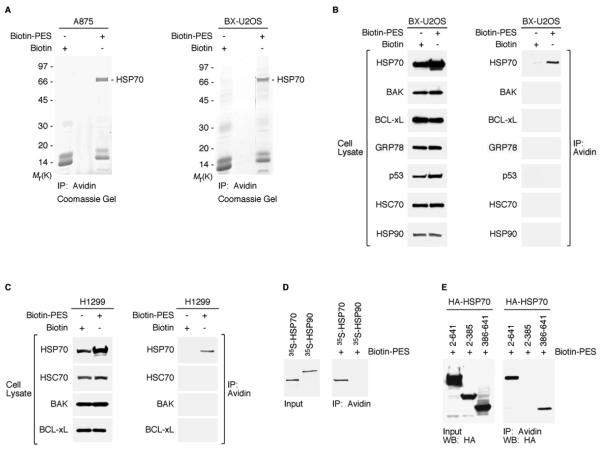
Figure 1. PES Binds to HSP70.
(A) Whole cell extracts (WCE) prepared from A875 and BX-U2OS cells, treated with Biotin or Biotin-PES, were captured using NeutrAvidin agarose resins. HSP70-family proteins were identified as the major product in the excised band of ~ 70 kDa shown in both Coomassie gels. HSP70 and HSC70 peptide sequences are shown in Figure S1B.
(B and C) WCE were prepared from the cell lines indicated following 24 h treatment with 20μM biotin or B-PES and examined for the expression of proteins indicated (left panel of B and C) by western blot analysis; note that different exposure times were used to visualize these proteins. B-PES-containing complexes were captured by NeutrAvidin Resins, and eluted following 100 mM DTT treatment. Immunoprecipitation-western blot (IP-WB) analysis using the indicated antibodies reveals interaction of B-PES with endogenous HSP70, but there is no detectable interaction with endogenous BAK, BCL-xL, GRP78, p53, HSC70, or HSP90 even after longer exposure times.
(D) In vitro evidence for an interaction between B-PES and HSP70. Full-length human HSP70 and human HSP90 proteins were in vitro translated in the presence of 35S-methionine, mixed with B-PES coupled to NeutrAvidin resins, and eluted using 100 mM DTT. The resulting DTT eluates were separated by polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by autoradiography.
(E) B-PES interacts with the C-terminal region of HSP70. H1299 cells were transfected with the indicated hemagglutinin (HA)-tagged constructs and exposed to B-PES. B-PES containing complexes were captured by NeutrAvidin Resins, eluted following 100 mM DTT treatment, and immunblotted with anti-HA antibody following SDS-PAGE.