Fig. 4.
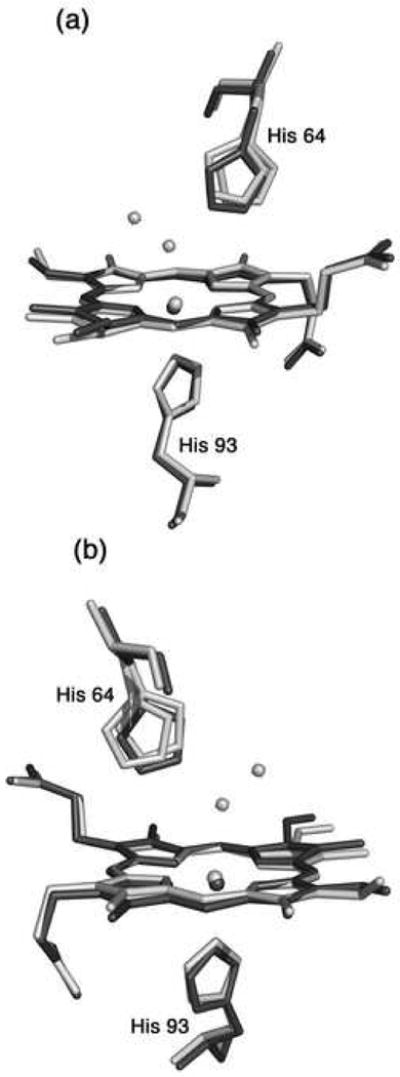
A superposition of the heme environments in the crystal structures of hh MnIIIMb(H2O) (this work, shown in light grey) and hh MnIIMb (this work, shown in dark grey) using a global Cα structural alignment, and shown from two different views. The Nε atoms of the His64 and His93 residues of MnIIMb are shifted from their major/minor positions in MnIIIMb(H2O) by 0.95/0.21 and 0.37 Å, respectively. Also, the MnII center is shifted 0.25 Å from its MnIII position. A meso carbon and its connecting pyrrole ring A are shifted towards the proximal side in MnIIMb relative to those in MnIIIMb(H2O).
