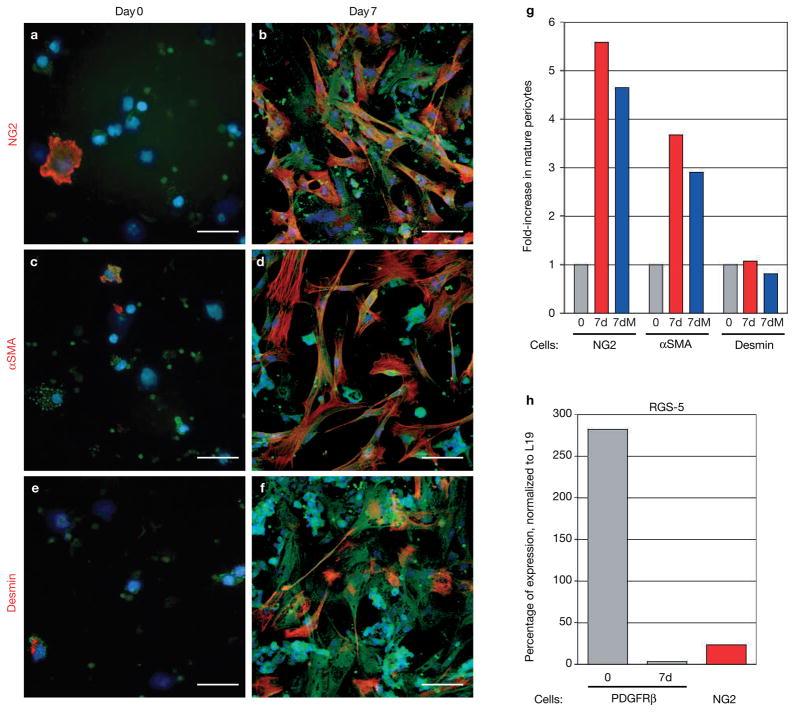
Figure 2.
PDGFRβ+ cells differentiate into mature pericytes in vitro. (a–f) PDGFRβ+ cells were isolated from tumours with a green fluorescent PDGFRβ antibody that was coated to magnetic beads and then cultured in vitro. Cells were immunolabelled with antibodies for the mature pericyte markers NG2 (a, b), αSMA (c, d) or desmin (e, f) immediately after the cells had settled onto the dish (day 0; a, c, e) or after 7 days in culture (day 7; b, d, f). (g) PDGFRβ+ cells that were positive for either NG2, αSMA or desmin, were counted at day 0 and day 7 to reveal the induction of mature pericytes. Whereas NG2+ and αSMA+ cells increased about fourfold after 7-day culture when compared with freshly isolated cells, desmin+ cells did not expand in number during the cell culture. Addition of the cell-cycle blocker mitomycin-C (5–10 μg ml−1; 7dM) during culture did not change the ratio of pericyte differentiation. (h) Quantitative Taqman RT–PCR analysis for RGS-5, a marker of developing and angiogenic pericytes, was performed on total RNA isolated from tumour-derived PDGFRβ+ (freshly isolated and cultured for 7 days) and NG2+ cells in Rip1Tag2 tumours. Expression levels were normalized to those of L19. RGS-5 levels were very high in isolated PDGFRβ+ cells (day 0) but markedly reduced in differentiated PDGFRβ+ cells in culture (day 7), and in mature tumour-derived NG2+ pericytes. Scale bars, 9 mm.