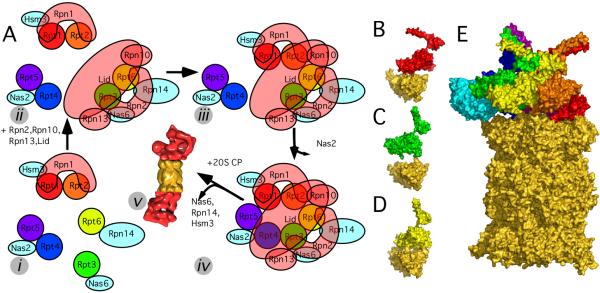
Figure 4.
Different assembly chaperones are required to position the AAA-ATPases on the CP. A: Model for the assembly process of the 26S proteasome. The assembly consists of at least five different steps: (i) Formation of different base-precursor complexes, each bound to a different chaperone (cyan). (ii) The lid and the remaining base subunits form a sub-complex with the AAA-ATPase pair Rpt3/Rpt6. (iii) An RP precursor lacking only Rpt4/Rpt5 forms. (iv) The Rpt4/Rpt5 dimer completes the RP and the chaperone Nas2 dissociates. (v) The three chaperones Nas6, Rpn14, and Hsm13 dissociate upon binding of RP and CP. B: Quaternary structure of Rpt1 (binds to assembly chaperone Hsm3) and the two adjacent CP alpha subunits (gold). C: Same for Rpt3 (binds to Nas6), and D: Rpt6 (Rpn14). E: Positioning Nas6 (PDB code 2DZN, [36]) on our CP-AAA-ATPase model does not lead to steric clashes with the CP.