TABLE 1.
Efficient, Mild Route to α-Fluoro Acrylonitriles
| entry | aldehyde | temp | product: yield,a%E/Z ratiob | =C(CN)F: δ (ppm);cJ (Hz) | lit:d yield, E/Z ratio |
|---|---|---|---|---|---|
| 1 | 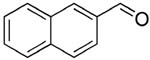 |
rt | 12: 98%, 15:85 | -122.1; 36.6 | -- |
| -78 °C | 12: 97%, 8:92 | -122.5; 15.3 | |||
| 2 | 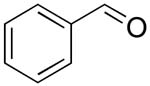 |
rt | 13: 93%, 19:81 | -121.9; 35.2 | 54%, 66:3310 |
| -122.6; 17.6 | 53%, 21:7911 | ||||
| 3 | 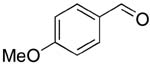 |
rt | 14: 95%, 16:84 | -126.2; 36.6 | 52%, 50:509 |
| -127.1; 18.3 | 50%, 32:6811 | ||||
| 4 | 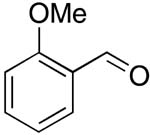 |
rt | 15: 91%, 37:63 | -122.3; 15.3 | -- |
| -78 °C | 15: 91%, 27:73 | -124.1; 36.6 | |||
| 5 | 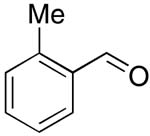 |
rt | 16: 94%, 17:83 | -120.4; 15.3 | -- |
| -123.0; 36.6 | |||||
| 6 | 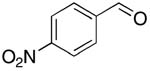 |
rt | 17: 72%, 17:83 | -115.3; 15.3 | 45%, 33:679 |
| -115.7; 33.6 | |||||
| 7 | 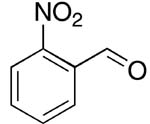 |
rt | 18: 60%, 15:85 | -117.0; 12.2 | 62%, 38:629 |
| -120.1; 30.5 | |||||
| 8 | 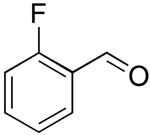 |
rt | 19: 91%, 16:84 | -118.8; 15.3 | -- |
| -119.8; 36.6 | |||||
| 9 | 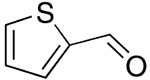 |
rt | 20: 96%, 17:83 | -122.8; 29.4 | -- |
| -129.0; 11.7 | |||||
| 10 | 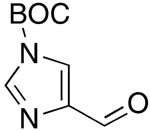 |
rt | 21: 59%, 18:82 | -117.9; 33.6 | -- |
| -125.3; 15.3 | |||||
| 11 | 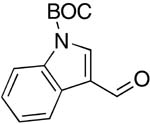 |
rt | 22: 86%, 20:80 | -119.2; 33.6 | -- |
| -125.9; 12.2 | |||||
| 12 | 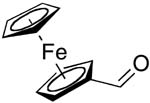 |
rt | 23: 92%, 17:83 | -128.5; 35.2 | -- |
| -129.6; 17.6 | |||||
| 13 | 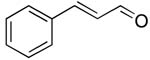 |
rt | 24: 81%, 17:83 | -126.6; 30.5 | 38%, 60:4010 |
| -127.7; 12.2 | 45%, 44:5611 | ||||
| 14 | n-heptyl-CHO | rt | 25: 97%, 23:77 | -123.9; 11.8 | Lit. data for n-heptanal:e 30%, 30:7010 50%, 26:7411 |
| -78 °C | 25: 90%, 16:84 | -125.8; 35.2 | |||
| 15 | 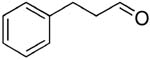 |
rt | 26: 80%, 23:77 | -122.6; 14.7 | 51%, 26:7411 |
| -78 °C | 26: 81%, 12:88 | -124.2; 32.3 | |||
| 16 | 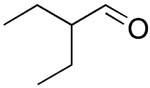 |
rt | 27: 76%, 15:85 | -122.8; 15.3 | -- |
| -78 °C | 27: 77%, 8:92 | -125.6; 30.5 |
Yields of isolated, purified products (reactions were performed under similar conditions but were not optimized for individual cases).
Relative ratio of diastereomers in the crude reaction mixture determined by 19F NMR prior to isolation. No change in ratio was observed after purification.
Referenced to CFCl3 as internal standard.
Where applicable, literature data and references are included for comparison.
Since no literature data for n-octanal are available, data for n-heptanal are included for comparison.
