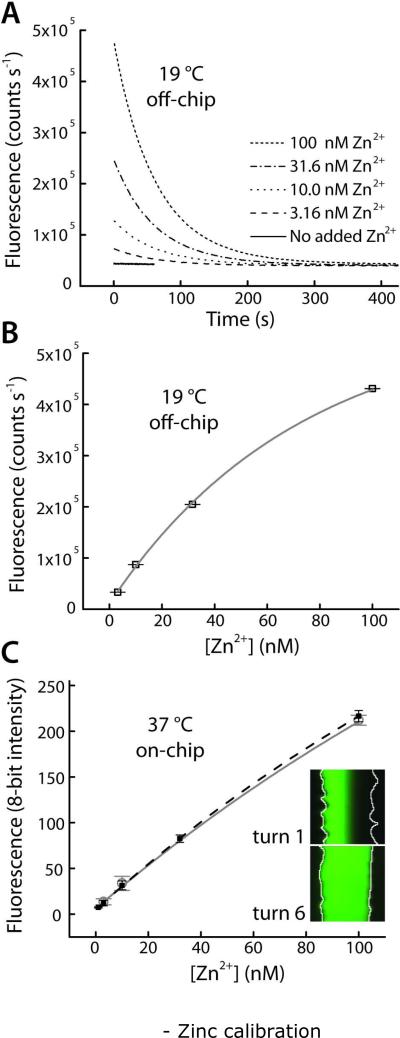
Figure 3.
Calibration for quantitative fluorescence detection of Zn2+ using FluoZin-3. (A) Ligand exchange rate of EDTA, pre-saturated with Ca2+ and Mg2+ ions in the imaging buffer, allowed brief observation of Zn2+ bursts. Collected at 19 °C with a spectrofluorometer (excitation 490 nm, emission 520 nm). (B) Data from (A) was fit to single exponential decay curves (lifetimes of 63.94 ± 0.91 s). Pre-exponential factors provided a calibration curve versus initial Zn2+ concentration (r2 = 0.9963). (C) On-chip calibration (filled black squares, dashed black line, r2 = 0.9999) and a typical daily calibration curves (open gray circles, gray line) collected in the microfluidic droplet sampling device at 37 °C. Inset shows that laminar separation of Zn2+ and Zn2+-free buffer at turn 1 of the microdevice was removed by diffusive mixing by turn 6, prior to droplet formation and calibration.