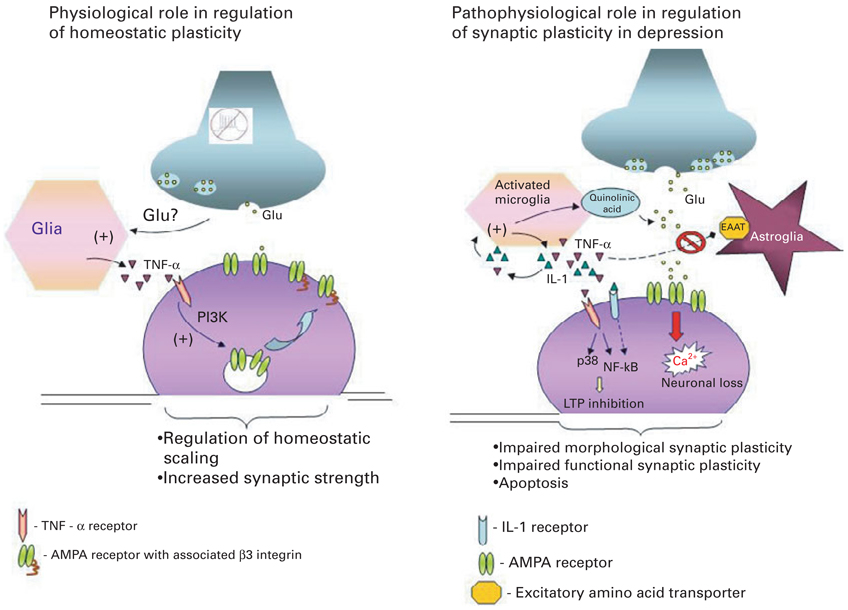
Fig. 1.
Dual role of pro-inflammatory cytokines in regulating synaptic plasticity. The diagram on the left depicts the critical role of constitutively expressed TNF-α in regulation of homeostatic synaptic plasticity in the normal brain. Decreased neuronal activity and consequently reduced glutamate release from axons is sensed by glia, which triggers release of TNF-α. TNF-α activates neuronal TNF-α receptors type I (TNFR1) leading to activation of the phosphoinositide-3 kinase (PI3K) pathway and up-regulation of specific adhesion molecule-β3 integrin, which in turn triggers α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor insertion to the membrane and increases synaptic strength. The diagram on the right depicts the various signalling cascades initiated by high pathophysiological levels of pro-inflammatory cytokines in the brain by activated microglia, which might underlie at least some aspects of the pathophysiology of depression. (1) TNF-α and IL-1 trigger production of quinolinic acid and release of glutamate by microglia; (2) TNF-α and IL-1 inhibit glutamate removal by astrocytes, leading to excess extracellular glutamate and neurotoxicity; (3) TNF-α acts via TNFR1 to up-regulate membrane expression of Ca-permeable AMPA receptor subunits, thus leading to increased Ca2+ influx and neuronal death; (4) TNFR1 activation coupled to activation of p38 and NF-κB pathways inhibits the early and late phases of LTP. These effects of pathophysiological levels of pro-inflammatory cytokines on synaptic plasticity at both morphological and functional levels might underlie the cognitive disturbances and impairments of memory seen in patients with depression.