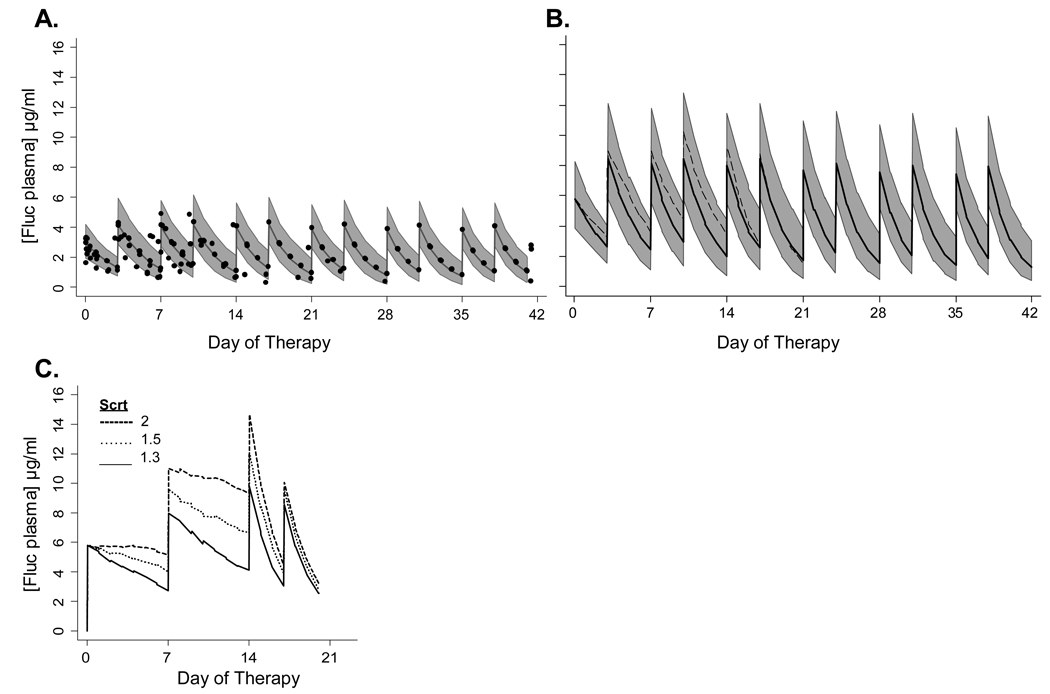
Figure 2.
Early prevention of invasive candidiasis in 23–29 week gestation infants. Predicted median and population variability range (10th–90th percentile) of fluconazole plasma concentrations from 100 simulated trials of preterm 23–29 week gestation infants treated with 3 mg/kg (A) or 6 mg/kg (B) twice weekly for 42 days starting in the first 5 days of life. Closed circles in (A) represent the observed fluconazole plasma concentrations in infants participating in a previous PK trial who received 3 mg/kgl. Dash line in B represents the median fluconazole concentration predicted in infants with serum creatinine of 1.2 mg/dl for first two weeks of therapy. (C) shows the predicted median fluconazole plasma concentrations in 23–29 week infants with renal insufficiency treated with 6 mg/kg weekly for first two weeks and then twice weekly beginning on day 14 when creatinine decreases to ≤1.0 mg/dl in simulation.