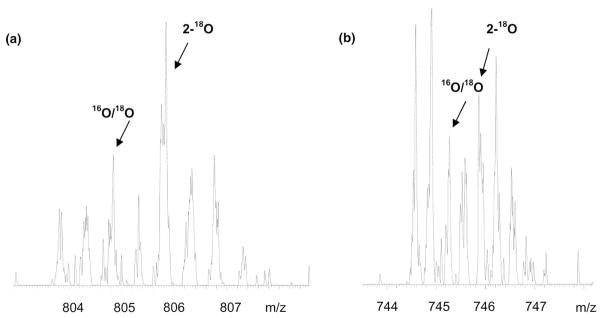
Figure 1.
(a) Zoom scan of the +2 charged peptide, VEADIAGHQEVLIR, from a myoglobin tryptic digest with an expected 18O/16O ratio of three. The unlabeled peptide (16O2) appears at m/z 803.8. The peaks from the doubly 18O labeled peptide and the singly labeled peptide are indicated by the arrows. The height of the M+2 peak shows clear enrichment from the singly labeled species. (b) Zoom scan of the +3 charged peptide, ESGSLSPEHGPVVVHCSAIGIGR, of PTP-1β from a tryptic digest of four standard proteins mixed with an expected 18O/16O ratio of one. The unlabeled peptide (16O2) appears at 744.6. This spectrum clearly illustrates the well resolved isotopic cluster provided by zoom scans for a +3 charged peptide. The peaks from the doubly 18O labeled peptide and the singly labeled peptide are indicated by arrows.