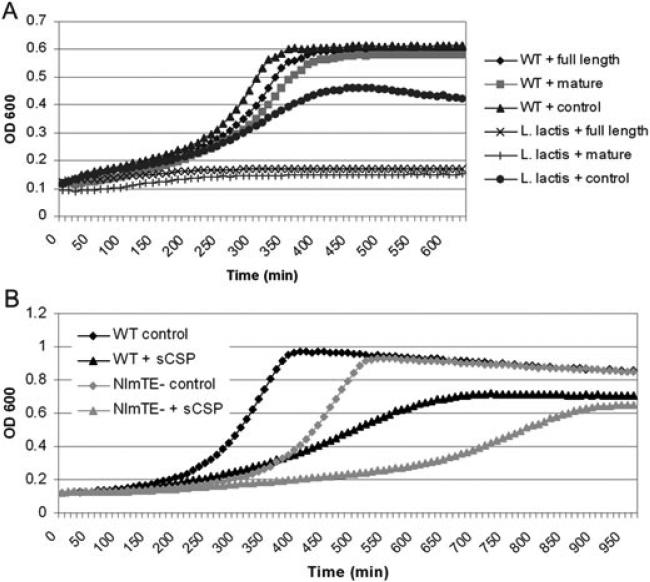
Fig. 6. CipB may act intracellularly.
A. Growth kinetics of S. mutans (WT) and L. lactis I6 in the presence of recombinant CipB (precursor and mature form). The full-length precursor peptide represents the intracellular form of the bacteriocin, including its leader sequence. The mature peptide represents the extracellular form of the bacteriocin, having been cleaved at the GG-motif. Importantly, the precursor peptide is equally effective against L. lactis, implying that export-dependent processing is not necessary for activity.
B. Effect of 2 μM sCSP (+) on the wild-type strain and a mutant defective in NlmTE, the ABC transporter responsible for export of CipB. The ΔnlmTE mutant was assayed over a range of sCSP concentrations and showed growth defects compared with the wild-type strain at all concentrations assayed (not shown), likely because of the intracellular accumulation of CipB. The ΔnlmTE mutant has a growth defect even in the absence of sCSP, possibly because of the accumulation of intracellular CipB induced by the high endogenous CSP concentration found in the overnight culture from which it was diluted.