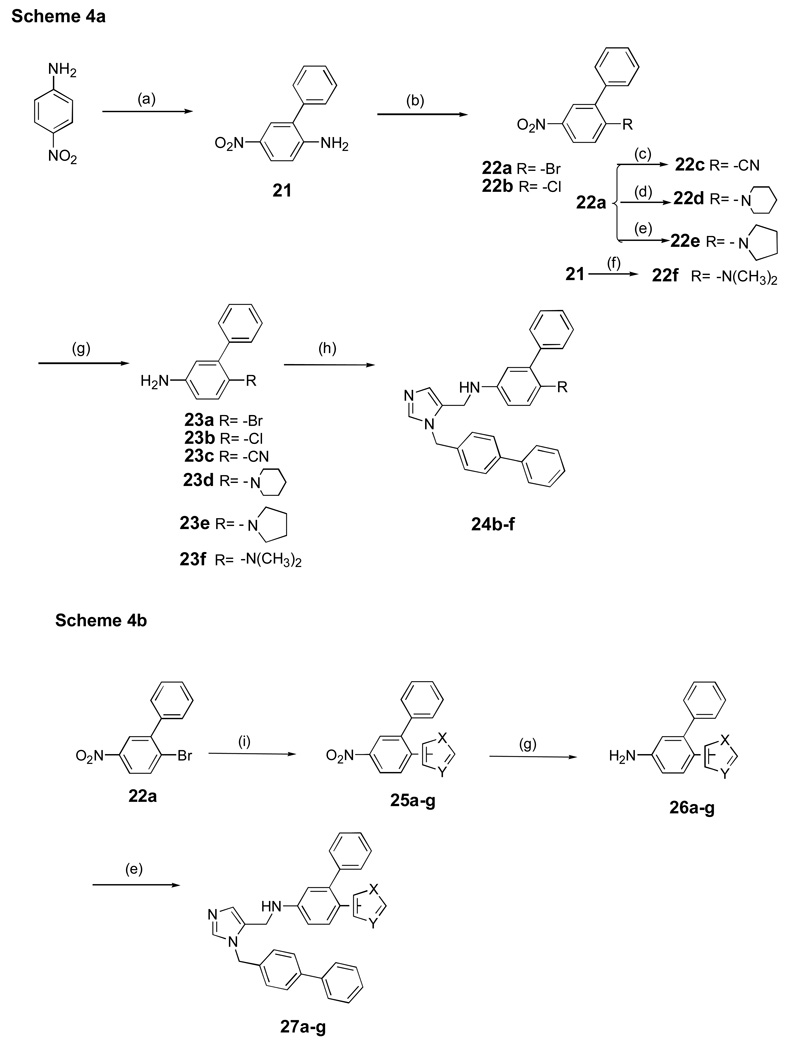
Scheme 4. aaSynthesis of dialkylimidazoles containing non-acyl functional groups.
baSynthesis of dialkylimidazoles containing heterocycles.
aReagents and conditions: (a) i) Br2, AcOH, r.t, 1h, 76%; ii) PhB(OH)2, Pd(OAc)2, K2CO3, acetone/H2O, reflux, 86% (b) NaNO2, H2SO4, AcOH i) CuBr2, HCl, 71%; ii) CuCl2, HCl, 75%; (c) Zn(CN)2, Pd(PPh3)4, DMF, 100 °C, overnight, 60–65%; (d) piperidine, 100 °C, 2 h, 90%; (e) pyrrolidine, 80 °C, 2–3 h, 80–90%; (f) HCHO, H2SO4, NaBH4 THF, 10–30°C, 0.5h, 65–70%; (g) SnCl2.2H2O , EtOAc, reflux, 6 h, 76 –80%; (h) AcOH, 5, MeOH, 4Å mol. sieves, NaBH3CN, overnight, r.t, 0–65% (i) Heterocycle, pd(PPh3)4, KOAc, DMAC, 160°C, overnight, 32–35%.