Table 2.
Activities of compounds showing general structure II against T. cruzi amastigotes (EC50) and Murine fibroblast cells (nM) (Scheme-2,Scheme-3,Scheme-4).
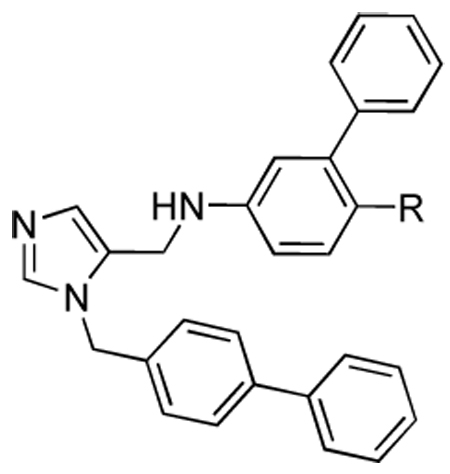 | |||
|---|---|---|---|
| Compound | R | EC50T. cruzi (nM) | EC50 Fibroblasts (nM) |
| 15a | COOEt | 10 | >10,000 |
| 15b | COOiPr | 50 | >10,000 |
| 15c | COOCy | 10 | >10,000 |
| 16a | C(=O)NHCH3 | 2.4, 3.0, 3.8, 5.1 | >1000 |
| 16b | C(=O)N(CH3)2 | 9.3, 14 | >1000 |
| 16c | C(=O)N(CH2)5 | 24, 39 | >1000 |
| 20a | C(=O)CH3 | 40 | >10,000 |
| 20b | C(=O)Et | 40 | >10,000 |
| 20c | C(=O)Pr | 40 | >10,000 |
| 24b | Cl | 6, 7 | >1000 |
| 24c | CN | 1.3, 1.8 | >1000 |
| 24d | 1-piperidine | 23, 35 | >1000 |
| 24e | 1-pyrrolidine | 22, 41 | >1000 |
| 24f | N(CH3)2 | 20, 26 | >1000 |
| 24g | OCH3 | 7, 9 | >1000 |
| 27a | 5-thiazole | 9, 9, 22, 28, 28 | >1,000 |
| 27b | 2-pyrrole | 100 | >10,000 |
| 27c | 2-benzofuran | 40 | >10,000 |
| 27d | 2-benzothiazole | 19,7,7,15,14,22,21 | >1,000 |
| 27e | 2-benzoxazole | 100 | >10,000 |
| 27f | 3-benzisoxazole | 10 | >10,000 |
| 27g | 3-anthranil | 50 | >10,000 |
