Figure 1.
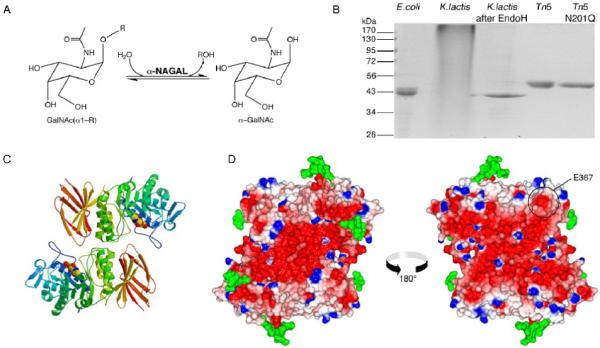
α-NAGAL reaction and overall structure
A. The reaction catalyzed by α-NAGAL. Both substrate and product are in the α anomeric configuration. B. Expression and purification of recombinant human α-NAGAL from different sources. Lane1: E. coli expressed α-NAGAL, Ni-affinity purified from inclusion bodies. Lanes 2 and 3: K. lactis expressed α-NAGAL before and after deglycosylation with Endo H. Lane 4 and 5: Tn5 expression of wild type α-NAGAL and N201Q α-NAGAL (with the 3rd carbohydrate site removed). C. A ribbon diagram of the human α-NAGAL dimer with the enzymatic product α-GalNAc in the active sites. D. An electrostatic map of the dimer showing contoured from -10 kT/e (red) to +10 kT/e (blue). Carbohydrates are shown in green. The left image is in the same orientation as in C, and the right image is rotated 180° about a vertical axis. The surface exposed residue E367 is circled.
