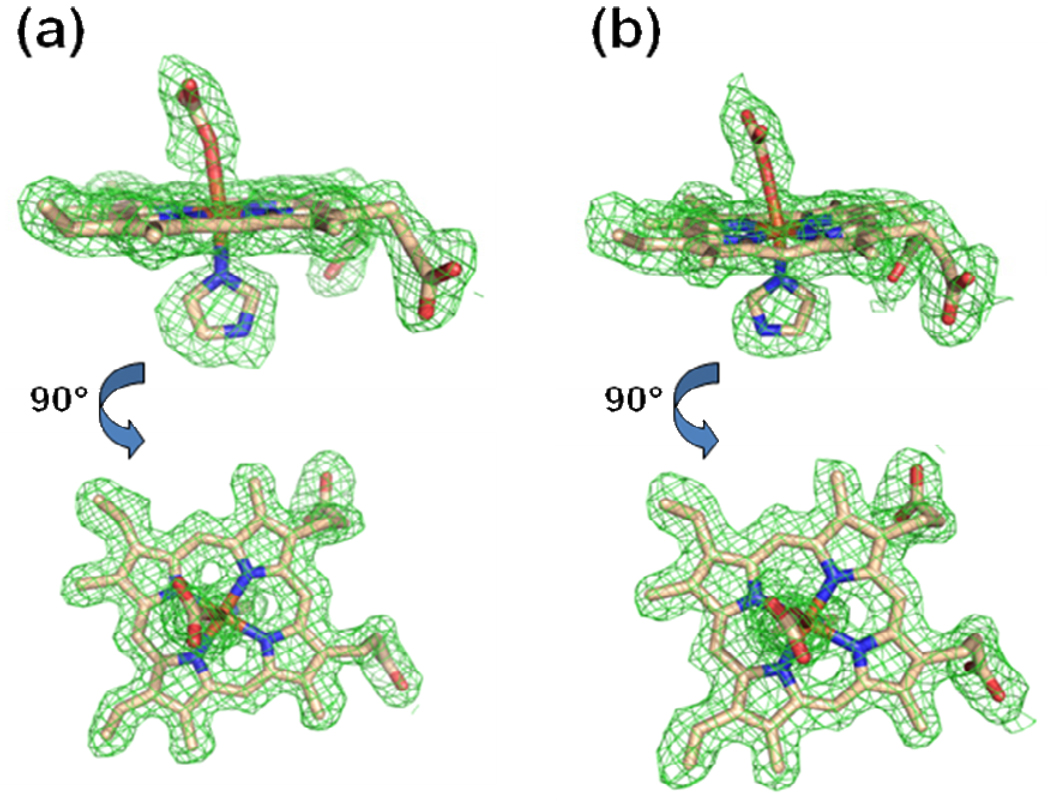
Figure 1.
Model and electron density revealing a metallated protoporphyrin IX bound within the active site of the A monomer for the wild-type human ferrochelatase enzyme treated with protoporphyrin IX and either Hg (Panel a) or Cd (Panel b). The model is shown in stick format and the 2Fo-Fc composite omit map (green cage) was generated using the simulated annealing protocol with 7% of the model omitted per cycle. The lower image in both panels is the same data shown in the top image rotated by 90°. Carbon, nitrogen, oxygen, and iron atoms are colored tan, blue, red, and orange respectively and the composite omit map is contoured at 1 σ.