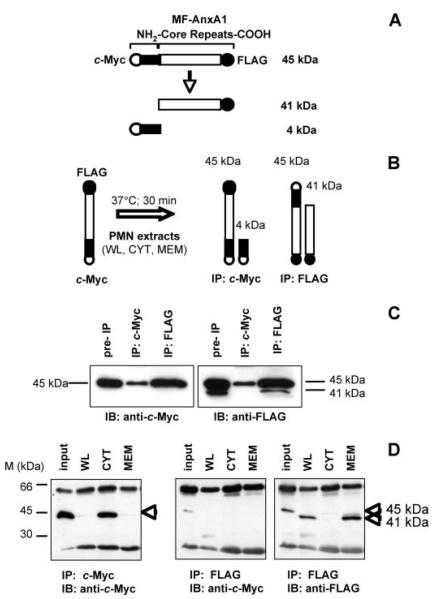
FIGURE 2. AnxA1-specific proteolytic activity resides in the membrane fraction of human PMN.
A, schematic representation of MF-AnxA1 and fragments resulting from proteolysis. B, depiction of the AnxA1 cleavage assay. PMN fractions (WL, CYT, MEM) were incubated with MF-AnxA1 for 30 min at 37 °C (left). Intact MF-AnxA1 could be recovered from the reaction mixture by analysis of the c-Myc IP (right), while N terminus-cleaved MF-AnxA1 could be recovered by IP for FLAG (far right). C, HEK-293T cells were transfected with MF-AnxA1. Cell lysates were IP for c-Myc or FLAG epitopes and immunoblotted with anti-c-Myc (left panel) or anti-FLAG (right panel). D, resting PMN-derived cell fractions were incubated with MF-AnxA1 (input) and assessed for AnxA1-specific proteolytic activity using the cleavage assay described above. Intact MF-AnxA1 was obtained by recovering the c-Myc IP and immunoblotting for c-Myc (left panel). N-terminal-cleaved MF-AnxA1 fragments were obtained by IP for FLAG and immunoblotting with anti-c-Myc (middle panel) or anti-FLAG (right panel) antibodies. Data are representative of five distinct preparations.