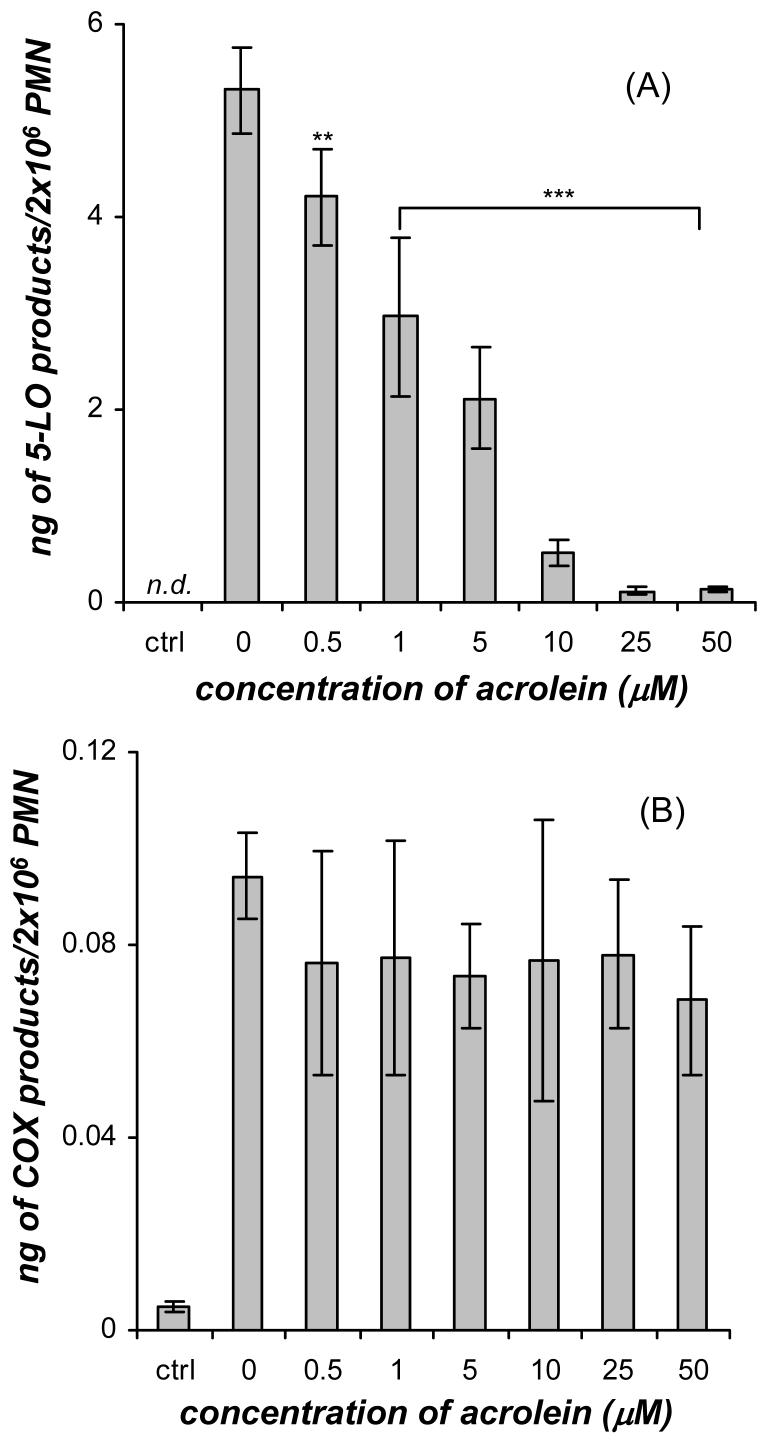
Figure 2.
Dose response of acrolein (0-50 μM) on the synthesis of (A) 5-LO products (20-OHLTB4, Δ6-trans-LTB4 isomers, LTB4, and 5-HETE) and (B) COX products (PGE2 and TXB2) formed in human neutrophils (PMN) primed with GM-CSF (1 nM, 30 min) and stimulated with fMLP (100 nM, 10 min) at 37°C. The GM-CSF primed cells were treated with acrolein for 3 min before fMLP was added. The control neutrophils were not treated with GM-CSF or fMLP. The 5-LO metabolites were analyzed by mass spectrometry and absolute quantities were calculated using relative abundances of the specific MRM relative to the deuterium labeled internal standard. Values (ng/2×106 cells) are expressed as mean ± S.E. (n = 5; ** p<0.01 and *** p<0.001).