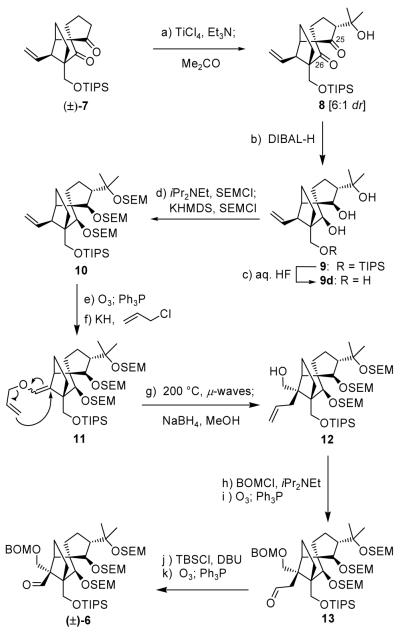
Scheme 1.
Construction of aldehyde (±)-6. Reagents and conditions: (a) TiCl4 (1.3 equiv), Et3N (3.0 equiv), acetone (10 equiv), CH2Cl2, −92 °C, 8 h; (b) DIBAL-H (5.0 equiv), PhMe, −78→0 °C, 30 min, 64 % for two steps; (c) aq. HF:THF (1:4), 25 °C, 18 h, 83 %; (d) iPr2NEt (20 equiv), SEMCl (6.0 equiv), nBu4NI (1.0 equiv), 50 °C, 24 h; KHMDS (3.0 equiv), SEMCl (5.0 equiv), Et3N (10 equiv), THF, −78→25 °C, 1 h, 96 % for the two steps; (e) O3, py (1.0 equiv), CH2Cl2:MeOH (1:1), −78 °C; then Ph3P (5.0 equiv), −78→25 °C, 1 h, 97 %; (f) KH (10 equiv), allyl chloride (30 equiv), HMPA (10 equiv), DME, −10→25 °C, 8 h, 95 %; (g) iPr2NEt (1.0 equiv), 1,2-dichlorobenzene, 200 °C (μ-waves), 20 min; then NaBH4 (10 equiv), MeOH, 1 h, 25 °C, 88 % for two steps; (h) BOMCl (10 equiv), iPr2NEt (30 equiv), nBu4NI (1.0 equiv), CH2Cl2, 50 °C 12 h; (i) O3, py (1.0 equiv), CH2Cl2:MeOH (1:1), −78 °C; then Ph3P (5.0 equiv), −78→25 °C, 1 h, 81 % for two steps; (j) TBSCl (10 equiv), DBU (20 equiv), CH2Cl2, 25 °C, 12 h; (k) O3, py (1.0 equiv), CH2Cl2:MeOH (1:1), −78 °C; then Ph3P (5.0 equiv), −78→25 °C, 1 h, 80 % for two steps. HMPA = hexamethylphosphoramide, DBU = 1,8-diazoicyclo[5.4.0]undec-7-ene.