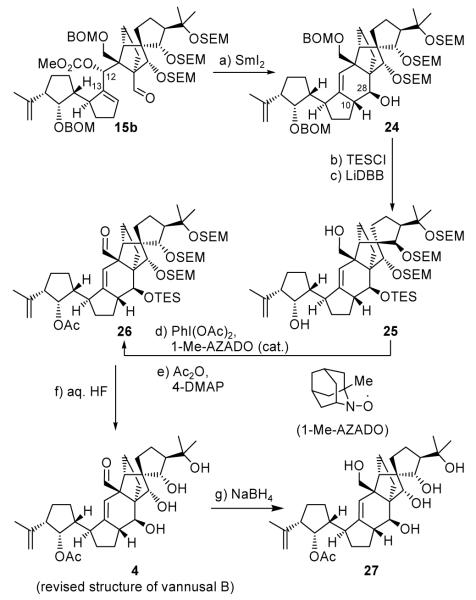
Scheme 4.
Completion of the revised structure of vannusal B (4). Reagents and conditions: (a) SmI2 (0.1 M in THF, 10 equiv), HMPA (30 equiv), THF, −20→25 °C, 30 min, 82 %; (b) KHMDS (5.0 equiv), TESCl (10 equiv), Et3N (10 equiv), THF, −78→25 °C, 20 min, 94 %; (c) LiDBB (excess), THF, −78→−50°C, 30 min, 83 %; (d) PhI(OAc)2 (2.0 equiv), 1-Me-AZADO (0.2 equiv), CH2Cl2, 25 °C, 18 h; (e) Ac2O (10 equiv), Et3N (20 equiv), 4-DMAP (1.0 equiv), CH2Cl2, 25 °C, 18 h, 87 % for two steps; (f) aq. HF:THF (1:4→1:3), 25 °C, 3 h, 85 %; (g) NaBH4 (20 equiv), MeOH, 20 min, 90 %.