Abstract
The administration and combination of a variety of chemotherapeutic agents for treatment of advanced or recurrent uterine cancer of different histologies is under current debate. Mixed Mullerian Tumors (MMTs), which contain both adenocarcinoma and sarcoma components, are the most rate histologic type and it is therefore difficult to conduct clinical trials to determine if they should be treated like endometrial adenocarinomas or like sarcomas. Flexible Heteroarotionoids (Flex-Hets) are a promising class of anti-cancer drugs with low toxicity that have demonstrated activity against a wide variety of cancer types, but their efficacy in uterine cancers is unknown. The objective of this study was to determine if cell lines established from endometrial carcinoma (HEC-1-A), uterine sarcoma (SK-UT-1) and MMT (MES-SA) cancers exhibit differential sensitivities to cisplatin, carboplatin, paclitaxel, docetaxel, doxorubicin and SHetA2, if SHetA2 can enhance sensitivity to the chemotherapeutic drugs and if SHetA2 exhibits a differential effect on uterine cancer cells in comparison to normal endometrial cells using a cytotoxicity assay. These cell lines did not differ in their sensitivities to platinum or taxel drugs. Doxorubicin was active against the sarcoma but not the adenocarcinoma or MMT cell lines. SHetA2 decreased the survival of all three cell lines, but did not enhance their sensitivities to the chemotherapeutic agents. Two of the three uterine cancer cell lines were more sensitive to SHetA2 in comparison to normal endometrial cells. In conclusion, doxorubicin appears to have a greater effect against sarcoma than other uterine histology types. SHetA2 is affective against uterine cancer cell lines, but does not enhance their sensitivities to chemotherapeutic agents.
Keywords: Uterine cancer, adenocarcinoma, MMT, sarcoma, doxorubicin, Flex-Het
INTRODUCTION
Cancer of the uterine corpus is estimated to be detected in 41,200 women per year and to cause 7,350 deaths per year in the United States (www.cancer.org). These deaths are due to cancers that are detected at advanced stage or that recur after primary therapy. Use of chemotherapy drugs for advanced or recurrent uterine cancer is currently being investigated. There are several types of uterine cancer that are being evaluated in clinical trials as separate cancers. About 95% of uterine cancers are adenocarcinomas, which arise from the endometrial epithelium, while about 2–4% are sarcomas, which arise from the uterine myometrium. Even more rare, are the uterine carcinosarcomas (also called Mixed Mullerian Tumors or MMTs), which contain both epithelial and myometrial components. There is current debate as to whether MMTs should be treated as adenocarcimas or as sarcomas[1].
The chemotherapeutic agents under investigation in uterine cancer include cisplatin, carboplatin, paclitaxel, docetaxel and doxorubicin. Cisplatin and carboplatin are platinum-containing compounds that cause DNA damage by binding and crosslinking DNA, paclitaxel and docetaxel are diterpenes that interfere with microtubules, doxorubicin is an anthracycline antibiotic that intercalates between DNA base pairs[2]. All of these compounds exert significant toxicity in the patients[1–9]. In patients with advanced or recurrent endometrial carcinoma, response rates of only 27–54% have been reported[5–7]. Uterine sarcomas and mixed mesodermal tumors are less well studied because of their rarity and have poor response to chemotherapy and poor patient prognosis[3,4].
Flexible-Heteroarotinoids (Flex-Hets) are a novel class of compounds that exhibit promising anti-cancer activity. The lead Flex-Het, called SHetA2, was effective against all cell lines representing 9 different cancer types of the National Cancer Institutes Human Tumor Cell Line Panel in vitro and against an ovarian cancer cell line in vivo[10]. Sensitivity of uterine cancers to SHetA2 is currently unknown. Although Flex-Hets were originally modeled after retinoids and retain the retinoid differentiation activity, they do not activate the nuclear retinoid receptors or exhibit the classical retinoid toxicities or teratogenicity[10–13]. In addition, Flex-Hets are potent inducers of apoptosis in cancer cells with less effect on normal cells[10,14].
The objective of this study was to determine if cell lines established from endometrial carcinoma (HEC-1-A), uterine sarcoma (SK-UT-1) and MMT (MES-SA) cancers exhibit differential sensitivities to cisplatin, carboplatin, paclitaxel, docetaxel, doxorubicin and SHetA2, if SHetA2 can enhance sensitivity to the chemotherapeutic drugs and if SHetA2 exhibits a differential effect on uterine cancer cells in comparison to normal endometrial cells.
MATERIALS AND METHODS
Cell lines and drugs
All cell lines were obtained from the American Tissue Culture Collection (ATCC). HEC-1-A was isolated from a stage IA endometrial cancer, MES-SA was established from a poorly differentiated uterine sarcoma, SK-UT- 1 cells were isolated from a grade III mixed mesodermal tumor (MMT) consistent with leiomyosarcoma. HEC-1-A and MES-SA were cultured in McCoy’s 5a medium (modified) with 1.5 mM L-glutamine adjusted to contain 2.2 g L−1 sodium bicarbonate and 10% fetal bovine serum (FBS). SK-UT-1 and primary endometrial cultures were maintained in Minimum Essential Medium (Eagle) (MEM) with 2 mM L-glutamine and Earle’s BSS adjusted to contain 1.5 g L−1 sodium bicarbonate, 0.1 mM non-essential amino acids and 1.0 mM sodium pyruvate and 10% FBS. Primary cultures of normal endometrial cells were isolated from menstrual blood collected from healthy volunteers under and Institutional Review Board (IRB) approved protocol as previously described[15].
Cisplatin, paclitaxel and doxorubicin were obtained from LKT Labs (St. Paul, MN). Carboplatin and docetaxel were obtained from Sigma (St. Louis, MO). The Flex-Het was synthesized and provided by K. Darrell Berlin (Oklahoma State University, Stillwater, OK) as previously described[13]. Cisplatin and carboplatin were dissolved in phosphate buffered saline and the others were dissolved in dimethyl sulfoxide (DMSO).
Cytotoxicity assay
Cells were plated at 2000 cells/well in 96 well microtiter plates and allowed to adhere overnight. A separate plate containing 2 fold concentrations of the final drug concentrations was prepared. A multichannel pipetor was used to add 50 μl of the 2X drug solutions to 50 μL of the media covering the plated cells. After 3 days of incubation, the media was removed and 50 μL each from a plate of 2X SHetA2 and a plate of 2X chemotherapeutic drugs were combined in the wells. After an additional 3 days of incubation with the drugs, the percentage of growth inhibition was measured using the CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay (Promega Corp., Madison, WI), which is composed of a novel tetrazolium compound that is metabolized by viable cells into a soluble formazan that can be quantitated by reading the OD. Each experiment was performed in triplicate and the three values for each treatment were averaged. To calculate survival, the average OD of the treated cultures was divided by that of the control cultures treated with solvent alone for the cisplatin treatments and retinoid alone for the combination treatments.
RESULTS AND DISCUSSION
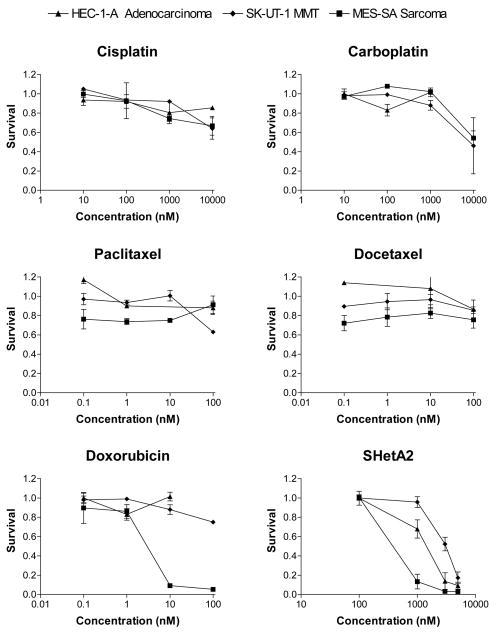
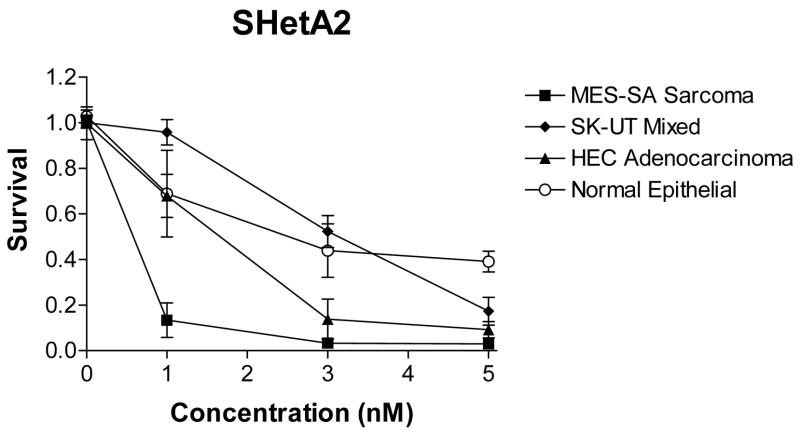
Three cell lines, HEC-1-A representing endometrial adenocarinoma, SK-UT-1 representing MMT and MES-SA representing uterine sarcoma were directly compared using a cytotocixity assay for their sensitivities to a variety of chemotherapeutic drugs and SHetA2, which were administered at physiologically achievable concentrations (Fig. 1). All three cell lines were fairly resistant to cisplatin, carboplatin, paclitaxel and docetaxel. Doxorubicin was effective against the uterine sarcoma, but not the adenocarcinoma or MMT. All three cell lines responded to SHetA2 with significantly different sensitivities. The sarcoma was the most sensitive, the MMT was the most resistant and the adenocarcinoma exhibited intermediate sensitivity. The primary endometrial cultures were more resistant to SHetA2 than the adenocarcinoma and sarcoma lines, but not the MMT (Fig. 2). Pre- and post treatment with SHetA2 did not alter the sensitivities of the cell lines to the chemotherapeutic agents (data not shown).
Fig. 1.
Effects of Chemotherapeutic Drugs and SHetA2 on Uterine Cancer Cell Lines of Different Histologies. The MTS assay was used to measure survival in cultures treated with a range of physiologically achievable drug concentrations. The x-axis is presented in log scale to allow comparison of the different drugs
Fig. 2.
Differential Effects of HetA2 on Uterine Cancer Cell Lines and Primary Endometrial Cells. The MTS assay was used to measure survival in cultures treated with a range of physiologically achievable drug concentrations. The x-axis is presented in linear scale to highlight the differential cell type sensitivities
This is the first report directly comparing the chemotherapeutic sensitivities of uterine cancer cell lines representing adenocarcinoma, MMT and sarcoma histologies. These cell lines did not differ in their sensitivities to platinum or taxel drugs. Doxorubicin was active against the sarcoma but not the adenocarcinoma or MMT cell lines. These results address the current debate as to whether MMTs should be treated as adenocarcinomas or sarcomas. The resistance of the adenocarinoma and MMT lines to doxorubicin would support treating patients with MMTs similar to the patients with adenocarinomas. This in vitro study however, suffers from the same limitations as the clinical studies in the rarity of uterine cancer cell lines and patients with MMT and sarcoma histologies. SHetA2 decreased the survival of all three cell lines at physiologically achievable concentrations[16], but did not enhance their sensitivities to the chemotherapeutic agents. Similar to the differential effect of SHetA2 previously observed in ovarian cancer cell lines in comparison to normal and benign cultures[10,13], two of the three uterine cancer cell lines were more sensitive to SHetA2 in comparison to normal endometrial cells.
CONCLUSION
Doxorubicin appears to have a greater effect against sarcoma than other uterine histology types. SHetA2 is affective against uterine cancer cell lines, but does not enhance their sensitivities to chemotherapeutic agents.
References
- 1.McCluggage WG. Uterine carcinosarcomas (malignant mixed Mullerian tumors) are metaplastic carcinomas. Intl J Gynecolog Cancer. 2002;12:687–90. doi: 10.1136/ijgc-00009577-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Falkner CA, McMeekin DS. Staging and Adjuvant Therapy for Endometrial Cancer. The Female Patient. 2005;30:18–26. [Google Scholar]
- 3.O’Meara AT. Uterine sarcomas: have we made any progress? Curr Opin Obstet Gynecol. 2004;16:1–4. doi: 10.1097/00001703-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Dusenbery KE, Potish RA, Argenta PA, Judson PL. On the apparent failure of adjuvant pelvic radiotherapy to improve survival for women with uterine sarcomas confined to the uterus. Am J Clin Oncol. 2005;28:295–300. doi: 10.1097/01.coc.0000156919.04133.98. [DOI] [PubMed] [Google Scholar]
- 5.Fleming GF, V, Brunetto L, Cella D, Look KY, Reid GC, Munkarah AR, Kline R, Burger RA, Goodman A, Burks RT. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: A Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159–66. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]
- 6.Fleming GF, V, Filiaci L, Bentley RC, Herzog T, Sorosky J, Vaccarello L, Gallion H. Phase III randomized trial of doxorubicin + cisplatin versus doxorubicin + 24-h paclitaxel + filgrastim in endometrial carcinoma: A Gynecologic Oncology Group study [see comment] Ann Oncol. 2004;15:1173–8. doi: 10.1093/annonc/mdh316. [DOI] [PubMed] [Google Scholar]
- 7.Thigpen JT, Brady MF, Homesley HD, Malfetano J, DuBeshter B, Burger RA, Liao S. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: A Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:3902–8. doi: 10.1200/JCO.2004.02.088. [DOI] [PubMed] [Google Scholar]
- 8.Gourley C, Al-Nafussi A, Abdulkader M, Smyth JF, Gabra H. Malignant mixed mesodermal tumours: biology and clinical aspects. Eur J Cancer. 2002;38:1437–46. doi: 10.1016/s0959-8049(02)00114-4. [DOI] [PubMed] [Google Scholar]
- 9.Kanjeekal S, Chambers A, Fung MFK, Verma S. Systemic therapy for advanced uterine sarcoma: A systematic review of the literature. Gynecolog Oncol. 2005;97:624–37. doi: 10.1016/j.ygyno.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 10.Benbrook DM, Kamelle SA, Guruswamy SB, Lightfoot SA, Hannafon B, Rutledge TL, Gould NS, Dunn ST, Berlin KD. Flexible heteroarotinoids (Flex-Hets) exhibit improved therapeutic ratios as anti-cancer agents over retinoic acid receptor antagonists. Inv New Drugs. 2005;23:417–428. doi: 10.1007/s10637-005-2901-5. [DOI] [PubMed] [Google Scholar]
- 11.Guruswamy S, Lightfoot S, Gold M, Hassan R, Berlin KD, Ivey RT, Benbrook DM. Effects of retinoids on cancerous phenotype and apoptosis in organotypic culture of ovarian carcinoma. J Nat Cancer Inst. 2001;93:516–525. doi: 10.1093/jnci/93.7.516. [DOI] [PubMed] [Google Scholar]
- 12.Mic FA, Molotkov A, Benbrook DM, Duester G. Retinoid activation of RAR but not RXR is sufficient for mouse embryonic development. Proc Natl Acad Sci. 2003;100:7135–7140. doi: 10.1073/pnas.1231422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Brown CW, Berlin KD, Dhar A, Guruswamy SB, Brown D, Gardner GJ, Birrer MJ, Benbrook DM. Synthesis of flexible sulfur-containing heteroarotinoids that induce apoptosis and reactive oxygen species with discrimination between malignant and benign cells. J Med Chem. 2004;47:999–1007. doi: 10.1021/jm030346v. [DOI] [PubMed] [Google Scholar]
- 14.Chun KH, Benbrook DM, Berlin KD, Hong WK, Lotan R. Induction of apoptosis in head and neck squamous cell carcinoma (HNSCC) cell lines by heteroarotinoids through a mitochondrial dependent pathway. Cancer Res. 2003;63:3826–3832. [PubMed] [Google Scholar]
- 15.Kamelle S, Sienko A, Benbrook DM. Retinoids and steroids regulate menstrual phase histological features in human endometrial organotypic cultures. Fertility and Sterility. 2002;78:596–602. doi: 10.1016/s0015-0282(02)03302-2. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Hua Y, Benbrook DM, Covey JM, Chan KK. High performance liquid chromatographic analysis and preclinical pharmacokinetics of the heteroarotinoid antitumor agent, SHetA2. Cancer Chemother Pharmacol. 2006 doi: 10.1007/s00280-006-0211-z. (In press) [DOI] [PubMed] [Google Scholar]