Abstract
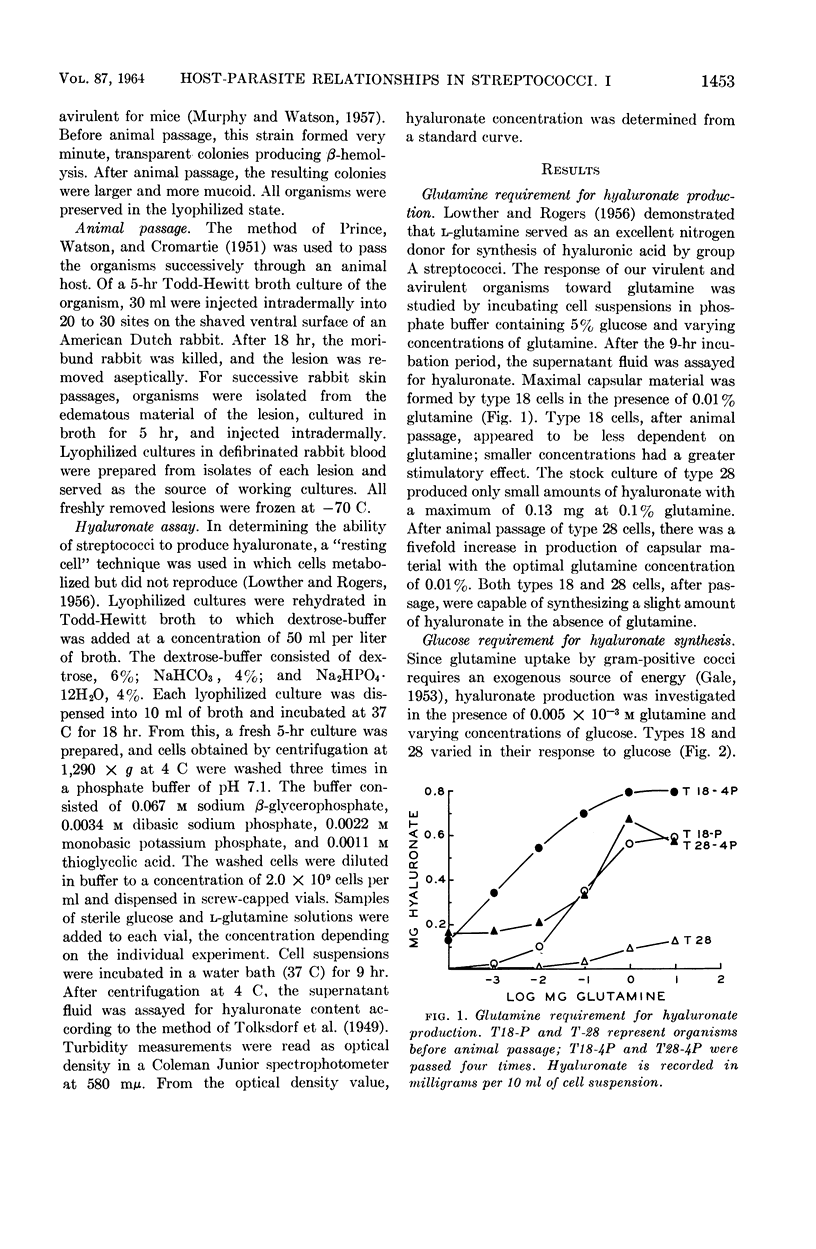
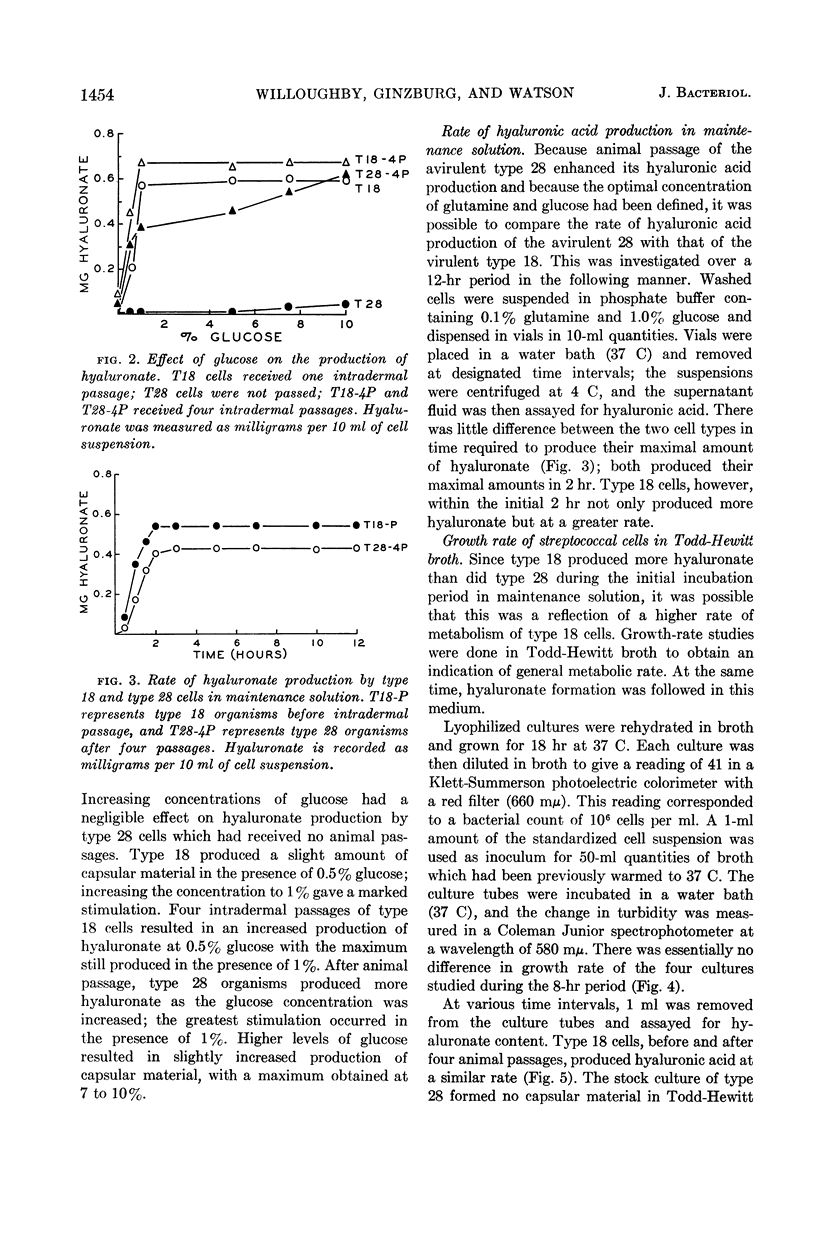
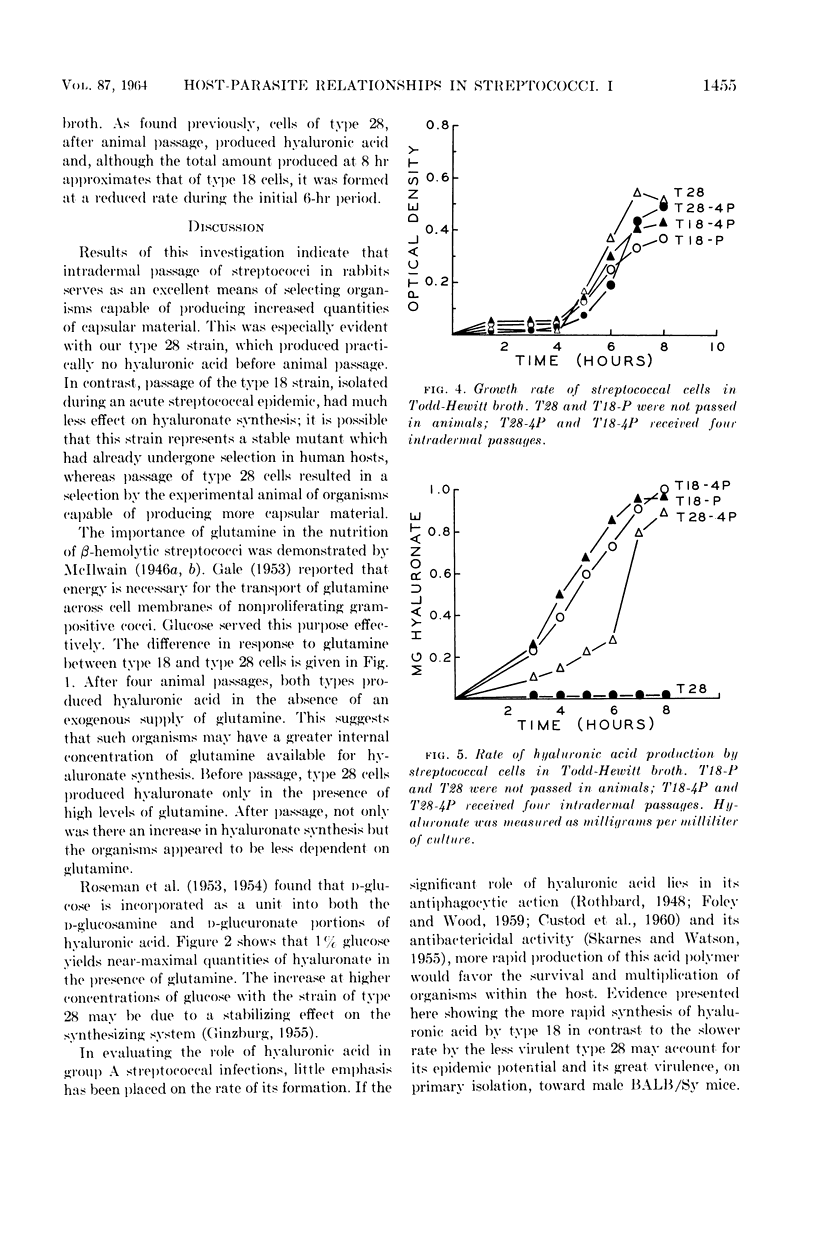
Willoughby, Donald S. (University of Minnesota, Minneapolis), Yael Ginzburg, and Dennis W. Watson. Host-parasite relationships among group A streptococci. I. Hyaluronic acid production by virulent and avirulent strains. J. Bacteriol. 87:1452–1456. 1964.—Intradermal passage of group A streptococcal strains in rabbits is an easy and effective technique for selecting organisms capable of producing increased amounts of hyaluronic acid. Glutamine was required for in vitro hyaluronate production, although the requirement varied with the organism tested. The virulent type 18 cells produced maximal amounts of hyaluronic acid in the presence of 0.01% glutamine compared with 0.1% for the more avirulent type 28; after animal passage, the glutamine requirement for type 28 decreased to 0.01%. Organisms selected by animal passage produced small quantities of hyaluronic acid in the absence of an exogenous source of glutamine. Glucose at a concentration of 1% gave the greatest stimulation for hyaluronate formation. Growth studies indicated similar rates of multiplication for types 18 and 28; yet, the rate of hyaluronate production under these conditions was much greater for the more virulent type 18. Because of these observations, it is suggested that the rate of hyaluronate production is a contributing factor in the virulence of type 18.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CUSTOD J. T., LYTLE R. I., JOHNSON B. H., FRANK P. F. Inter-dependence of hyaluronic acid and M protein in streptococcal aerosol infections in mice. Proc Soc Exp Biol Med. 1960 Apr;103:751–753. doi: 10.3181/00379727-103-25659. [DOI] [PubMed] [Google Scholar]
- FOLEY M. J., WOOD W. B., Jr Studies on the pathogenicity of group A streptococci. II. The antiphagocytic effects of the M protein and the capsular gel. J Exp Med. 1959 Oct 1;110:617–628. doi: 10.1084/jem.110.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALE E. F. Assimilation of amino acids by Gram-positive bacteria and some actions of antibiotics thereon. Adv Protein Chem. 1953;8:285–391. doi: 10.1016/s0065-3233(08)60094-7. [DOI] [PubMed] [Google Scholar]
- JOHNSON B. H., FURRER W. Hyaluronic acid: a required component of beta hemolytic streptococci for infecting mice by aerosol. J Infect Dis. 1958 Sep-Oct;103(2):135–141. doi: 10.1093/infdis/103.2.135. [DOI] [PubMed] [Google Scholar]
- Kuttner A. G., Krumwiede E. OBSERVATIONS ON THE EFFECT OF STREPTOCOCCAL UPPER RESPIRATORY INFECTIONS ON RHEUMATIC CHILDREN: A THREE-YEAR STUDY. J Clin Invest. 1941 May;20(3):273–287. doi: 10.1172/JCI101220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWTHER D. A., ROGERS H. J. The role of glutamine in the biosynthesis of hyaluronate by streptococcal suspensions. Biochem J. 1956 Feb;62(2):304–314. doi: 10.1042/bj0620304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY W. H., Jr, WATSON D. W. Susceptibility of inbred mice to group A streptococcal infection. Proc Soc Exp Biol Med. 1957 Apr;94(4):755–758. doi: 10.3181/00379727-94-23076. [DOI] [PubMed] [Google Scholar]
- McIlwain H. The metabolism and functioning of vitamin-like compounds: 1. Ammonia formation from glutamine by haemolytic streptococci; its reciprocal connexion with glycolysis. Biochem J. 1946;40(1):67–78. [PMC free article] [PubMed] [Google Scholar]
- McIlwain H. The metabolism and functioning of vitamin-like compounds: 3. Products of the decomposition of glutamine during streptococcal glycolysis. Biochem J. 1946;40(4):460–464. [PMC free article] [PubMed] [Google Scholar]
- ROSEMAN S., LUDOWIEG J., MOSES F. E., DORFMAN A. The biosynthesis of hyaluronic acid by group A Streptococcus. II. Origin of the glucuronic acid. J Biol Chem. 1954 Feb;206(2):665–669. [PubMed] [Google Scholar]
- ROSEMAN S., MOSES F. E., LUDOWIEG J., DORFMAN A. The biosynthesis of hyaluronic acid by group A Streptococcus. I. Utilization of 1-C14-glucose. J Biol Chem. 1953 Jul;203(1):213–225. [PubMed] [Google Scholar]
- ROSENDAL K., FABER V. Streptococcalhyaluronidase. III. The effect of penicillin on the production of hyaluronic acid and hyaluronidase by hemolytic streptococci (type 24, group A). Acta Pathol Microbiol Scand. 1955;36(3):263–273. [PubMed] [Google Scholar]
- SKARNES R. C., WATSON D. W. The inhibition of lysozyme by acidic polymers from pathogenic bacteria. J Bacteriol. 1955 Jul;70(1):110–112. doi: 10.1128/jb.70.1.110-112.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]


