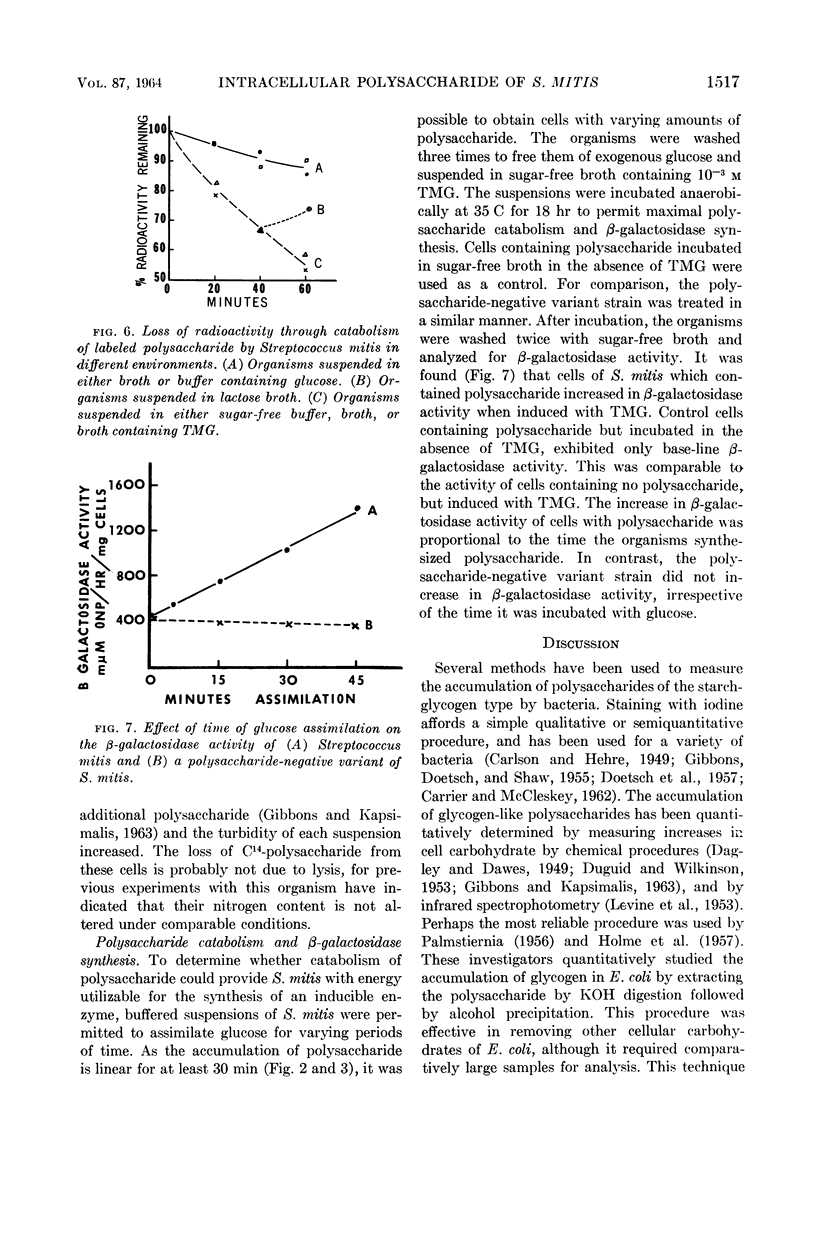
Abstract
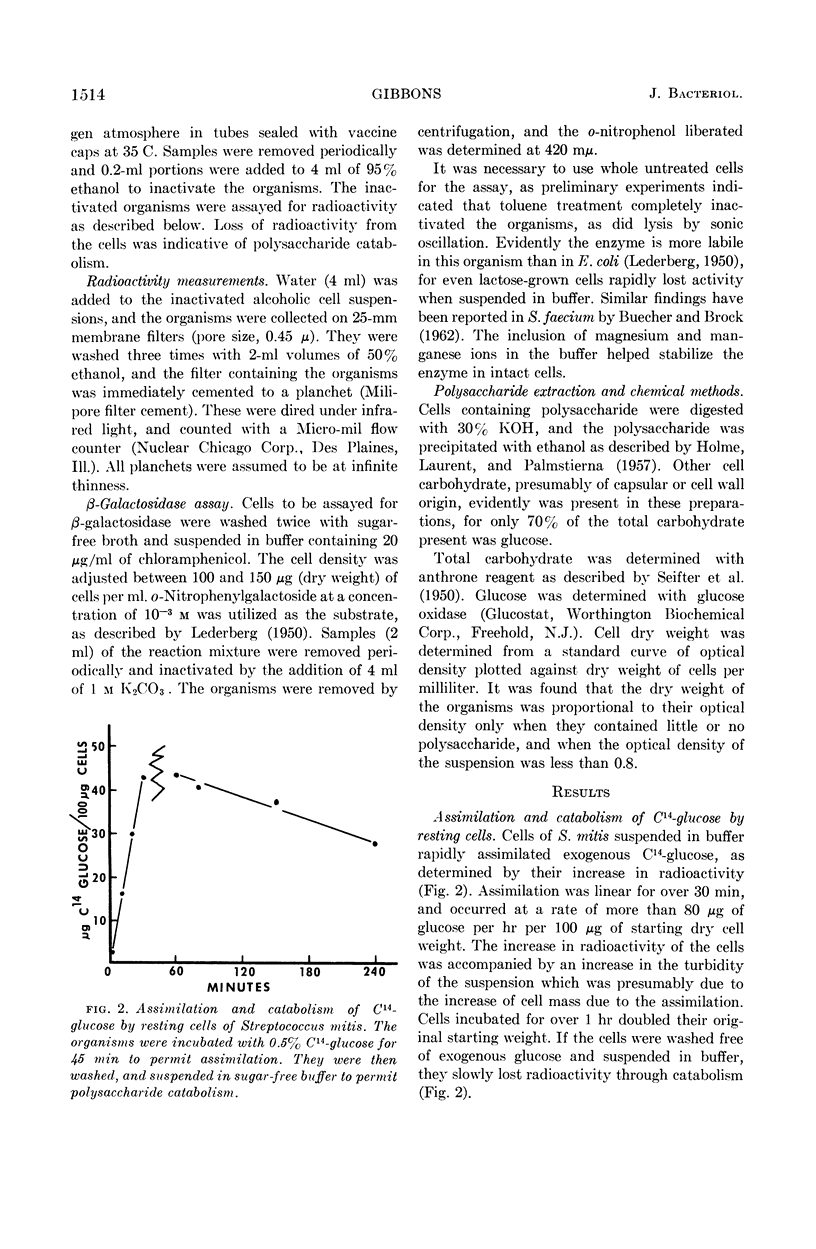
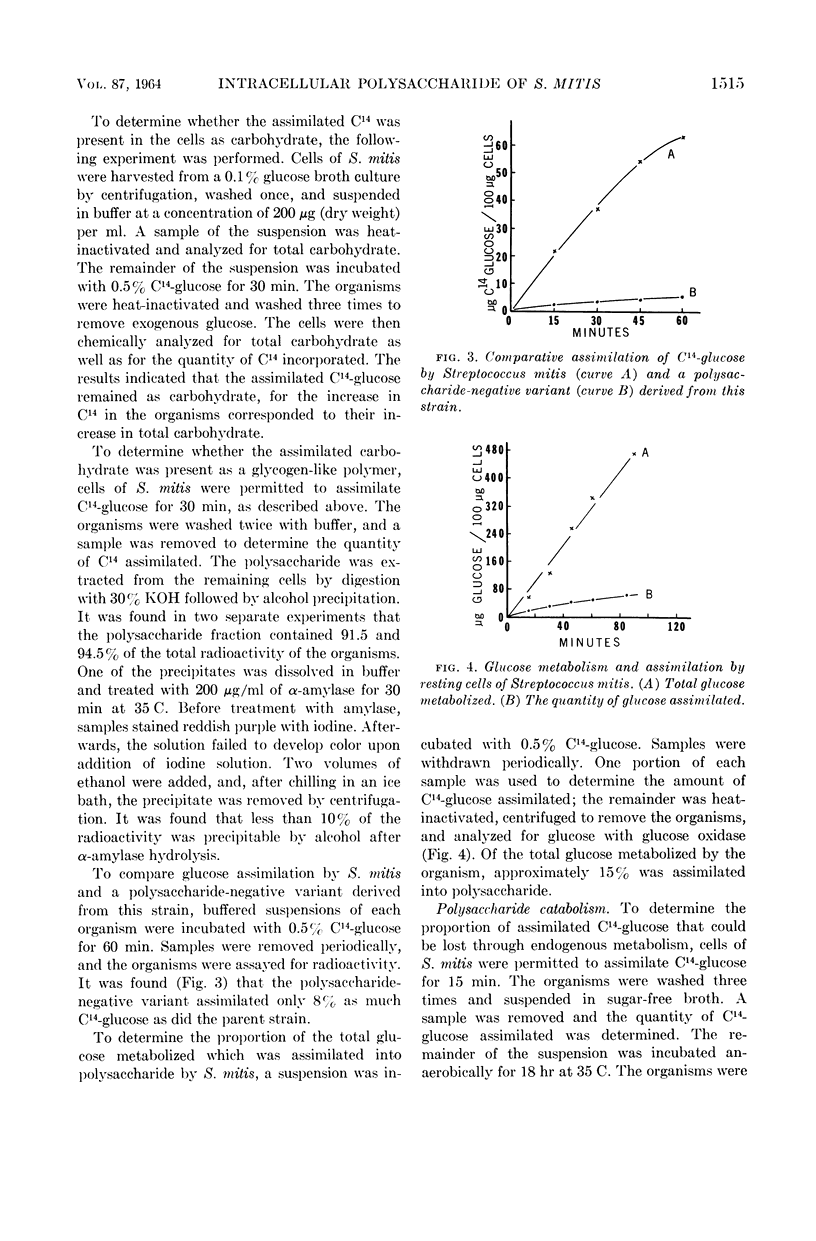
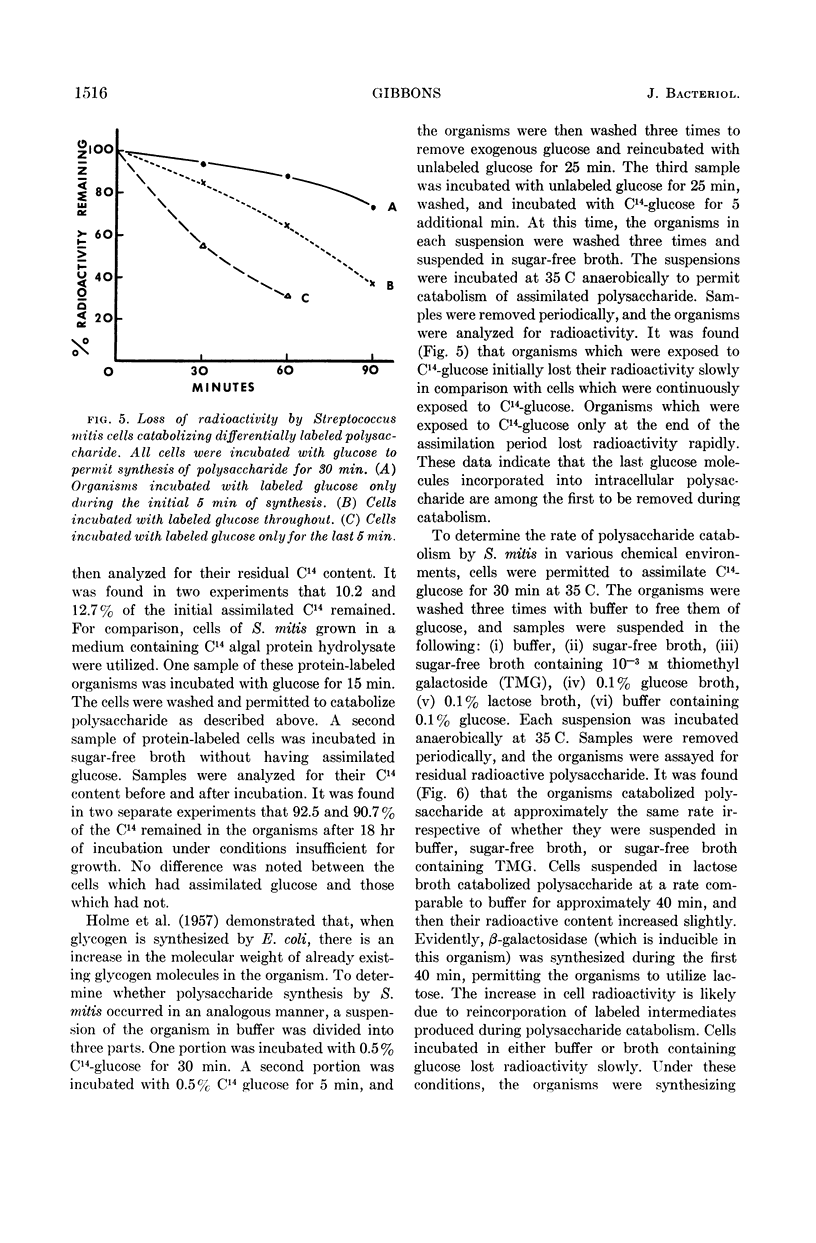
Gibbons, R. J. (Forsyth Dental Center, Boston Mass.). Metabolism of intracellular polysaccharide by Streptococcus mitis and its relation to inducible enzyme formation. J. Bacteriol. 87:1512–1520. 1964.—The synthesis and catabolism of an intracellular iodine staining polysaccharide produced from glucose by Streptococcus mitis was investigated. Approximately 15% of the total glucose metabolized by buffered suspensions of S. mitis was assimilated. Over 90% of the assimilated glucose was converted into a polysaccharide of the glycogen-amylopectin type. Use of uniformly labeled C14-glucose provided a convenient method for determining polysaccharide accumulation in this organism. Glucose assimilation occurred at a rate of over 80 μg of glucose per hr per 100 μg of starting dry cell weight. Prolonged assimilation produced cells containing over 50% polysaccharide on a dry weight basis. Accumulated polysaccharide was catabolized at the same rate when the organism was suspended in buffer, sugar-free broth, or sugar-free broth containing thiomethyl galactoside. Metabolic intermediates produced from polysaccharide catabolism did not markedly repress inducible enzyme synthesis. The last glucose molecules incorporated into polysaccharide were among the first molecules to be removed during catabolism. Catabolism of polysaccharide provides S. mitis with energy in a utilizable form, for cells containing polysaccharide increased in β-galactosidase activity when induced with thiomethyl galactoside in the absence of an exogenous energy source. Cells devoid of polysaccharide, and a polysaccharide-negative variant of S. mitis did not increase in β-galactosidase activity when induced in a similar manner. It appears that the intracellular polysaccharide is the sole substrate for the endogenous metabolism of S. mitis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAGLEY S., DAWES E. A. Factors influencing the polysaccharide content of Escherichia coli. Biochem J. 1949;45(3):331–337. doi: 10.1042/bj0450331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., JOHNSON A. R. The relation between lipid and polysaccharide contents of Bact. coli. Biochim Biophys Acta. 1953 May;11(1):158–159. doi: 10.1016/0006-3002(53)90020-1. [DOI] [PubMed] [Google Scholar]
- DANFORTH W. F., WILSON B. W. The endogenous metabolism of Euglena gracilis. J Gen Microbiol. 1961 Jan;24:95–105. doi: 10.1099/00221287-24-1-95. [DOI] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. The endogenous metabolism of microorganisms. Annu Rev Microbiol. 1962;16:241–264. doi: 10.1146/annurev.mi.16.100162.001325. [DOI] [PubMed] [Google Scholar]
- DOETSCH R. N., HOWARD B. H., MANN S. O., OXFORD A. E. Physiological factors in the production of an iodophilic polysaccharide from pentose by a sheep rumen bacterium. J Gen Microbiol. 1957 Feb;16(1):157–168. doi: 10.1099/00221287-16-1-156. [DOI] [PubMed] [Google Scholar]
- DUGUID J. P., WILKINSON J. F. The influence of cultural conditions on polysaccharide production by Aerobacter aerogenes. J Gen Microbiol. 1953 Oct;9(2):174–189. doi: 10.1099/00221287-9-2-174. [DOI] [PubMed] [Google Scholar]
- EATON N. R. Endogenous respiration of yeast. I. The endogenous substrate. Arch Biochem Biophys. 1960 May;88:17–25. doi: 10.1016/0003-9861(60)90192-2. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. J., KAPSIMALIS B. Synthesis of intracellular iodophilic polysaccharide by Streptococcus mitis. Arch Oral Biol. 1963 May-Jun;8:319–329. doi: 10.1016/0003-9969(63)90024-4. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. J., SOCRANSKY S. S. Intracellular polysaccharide storage by organisms in dental plaques. Its relation to dental caries and microbial ecology of the oral cavity. Arch Oral Biol. 1962 Jan-Feb;7:73–79. doi: 10.1016/0003-9969(62)90050-x. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. J., SOCRANSKY S. S., SAWYER S., KAPSIMALIS B., MACDONALD J. B. The microbiota of the gingival crevice area of man. II. The predominant cultivable organisms. Arch Oral Biol. 1963 May-Jun;8:281–289. doi: 10.1016/0003-9969(63)90020-7. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E. POLYSACCHARIDE STORAGE AND GROWTH EFFICIENCY IN RUMINOCOCCUS ALBUS. J Bacteriol. 1963 Oct;86:848–854. doi: 10.1128/jb.86.4.848-854.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZEN H. M., STETTEN D., Jr, STETTEN M. R. Metabolic inhomogeneity of glycogen as a function of molecular weight. J Biol Chem. 1956 Oct;222(2):587–599. [PubMed] [Google Scholar]
- LEDERBERG J. The beta-d-galactosidase of Escherichia coli, strain K-12. J Bacteriol. 1950 Oct;60(4):381–392. doi: 10.1128/jb.60.4.381-392.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE S., STEVENSON H. J., TABOR E. C., BORDNER R. H., CHAMBERS L. A. Glycogen of enteric bacteria. J Bacteriol. 1953 Dec;66(6):664–670. doi: 10.1128/jb.66.6.664-670.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIFTER S., DAYTON S. The estimation of glycogen with the anthrone reagent. Arch Biochem. 1950 Jan;25(1):191–200. [PubMed] [Google Scholar]