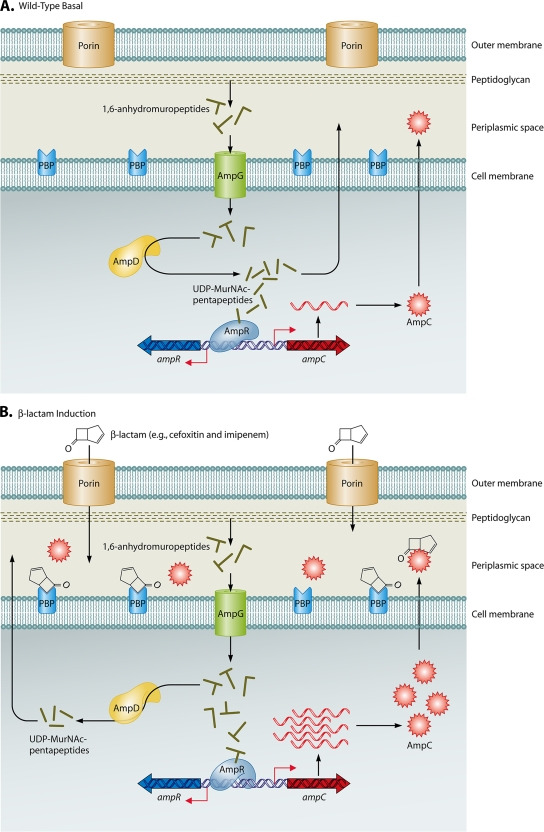
FIG. 3.
Mechanisms involved in regulation of ampC expression. These figures represent the current knowledge obtained from studies with members of the Enterobacteriaceae and appear to parallel events in P. aeruginosa. (A) Wild-type basal expression of ampC. During normal cell wall recycling, 1,6-anhydromuropeptides are removed from the cell wall and transported into the cytoplasm via the AmpG permease. The 1,6-anhydromuropeptides are cleaved by AmpD to generate free tripeptides, which are later converted into UDP-MurNAc-pentapeptides. UDP-MurNAc-pentapeptide interacts with AmpR bound to the ampR-ampC intergenic region, creating a conformation that represses transcription of ampC. Low basal levels of AmpC are produced, and the enzyme is localized to the periplasmic space. (B) β-Lactam induction of ampC expression. Inducing β-lactams, such as cefoxitin and imipenem, cross the outer membrane through porins, enter the periplasmic space, and interact with target PBPs. An increase in pools of 1,6-anhydromuropeptides is observed, and AmpD is unable to efficiently process the higher levels of cell wall fragments. The anhydro-MurNAc-peptides (inducing peptides) replace UDP-MurNAc-pentapeptides (suppressing peptides) bound to AmpR, causing a conformational change in the protein. AmpR is converted into a transcriptional activator, ampC is expressed at higher levels, and levels of AmpC increase in the periplasmic space. When the amount of β-lactam decreases below “inducing levels,” the cytoplasmic pool of anhydro-MurNAc-peptides also decreases, and AmpD is able to efficiently cleave these peptides, restoring wild-type ampC expression, as shown in panel A. (C) AmpD-associated derepression of ampC expression. Mutations leading to the inactivation of AmpD or decreased expression of ampD impair the processing of cell wall recycled products and lead to increased levels of anhydro-MurNAc-peptides (inducing peptides) in the cytoplasm. As a result, the binding of inducing peptides to AmpR is favored, AmpR is “locked” in a conformation for transcriptional activation of ampC expression, and high-level constitutive expression of ampC is observed.