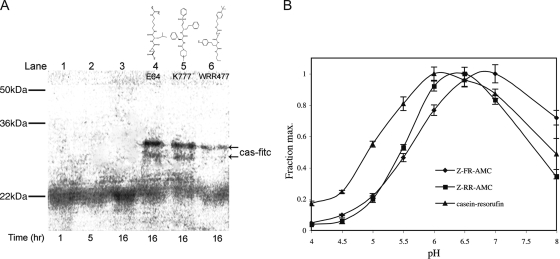
FIG. 4.
Giardia cysteine proteases degraded endocytosed proteins and were optimally active at a higher pH than their mammalian orthologues. (A) Cysteine proteases degraded endocytosed casein-FITC (cas-fitc). Cells were pulsed for 30 min with substrate and chased with fresh medium for the times indicated, and the cell lysates were examined. Lanes 1, 2, and 3 show loss of substrate in the cells following 1, 5, and 16 h of incubation. In the presence of three known cysteine protease inhibitors (10 μM) (lanes 4, 5, and 6), casein degradation was blocked (arrows). (B) pH profile of G. lamblia cathepsin B-like cysteine protease activity against the fluorogenic substrates Z-FR-AMC and Z-RR-AMC and against the macromolecular substrate casein-resorufin. The pH optimum was 6 to 7 for activities against all substrates. The error bars indicate standard errors.