FIG. 2.
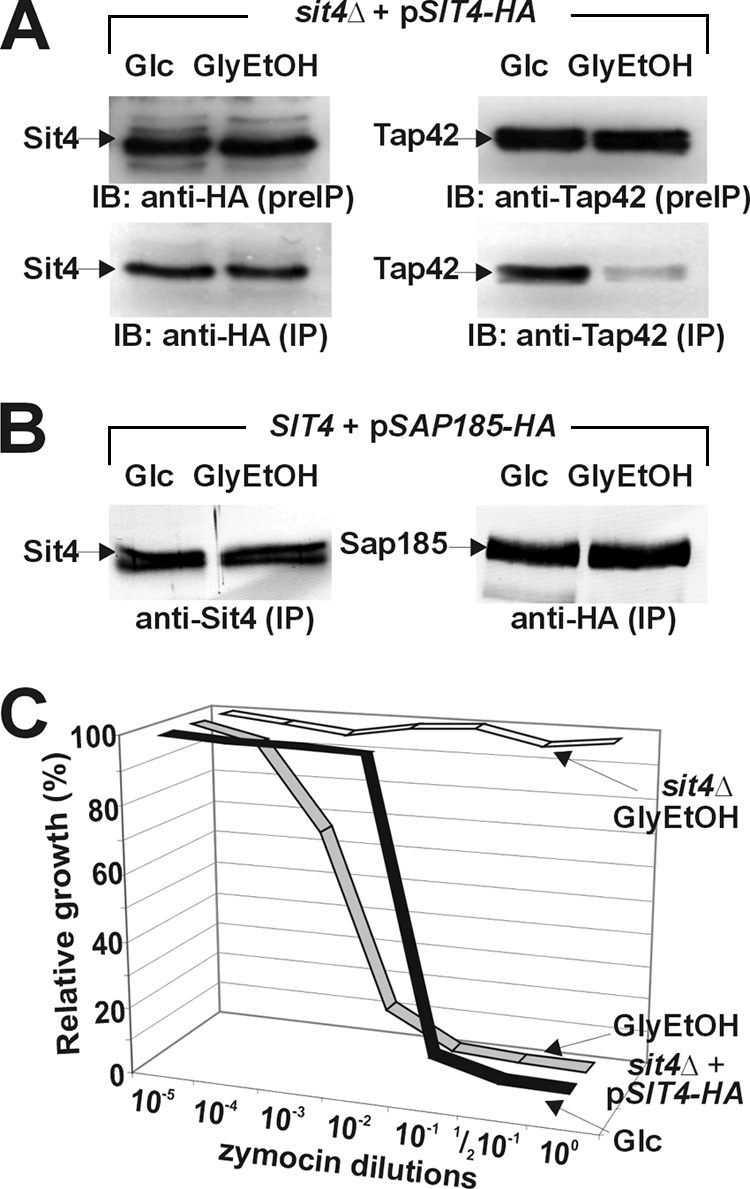
Zymocin inhibition is independent of Sit4/Tap42 interaction. (A) TOR pathway downregulation induces Sit4/Tap42 complex dissociation. Prior to immune precipitation (preIP), protein extracts prepared from Sit4-HA-expressing cells grown under glucose (Glc) or glycerol-ethanol (GlyEtOH), conditions, which promote or downregulate, respectively, TOR pathway signaling, were monitored for expression of Sit4-HA and Tap42 by immune blotting (IB) using anti-HA (top left) and anti-Tap42 (top right) antibodies. Immune precipitates (IP) obtained with the anti-HA antibody were next analyzed in Western blots using anti-HA and anti-Tap42 antibodies to check for the content of Sit4-HA and Tap42, respectively (indicated by arrows). (B) TOR pathway downregulation leaves Sit4/Sap185 interaction unaltered. SAP185-HA-expressing strains were grown as described for panel A and subjected to immune precipitation using anti-HA antibodies. The precipitates were analyzed by anti-Sit4 and anti-HA antibodies in immune blots to monitor the content of Sit4 and Sap185-HA. Note that TOR pathway downregulation did not affect Sit4/Sap185 interaction. (C) TOR pathway downregulation enhances zymocin inhibition. Under TOR-promoting (Glc) or TOR-downregulating (GlyEtOH) conditions, sit4Δ cells carrying SIT4-HA on a single-copy vector were grown in the presence of the indicated zymocin dilutions. Growth is expressed in relation (percentage of optical density at 600 nm) to that of zymocin-minus controls. sit4Δ cells carrying an empty vector and grown under TOR-downregulating conditions were included as a zymocin-resistant control.
