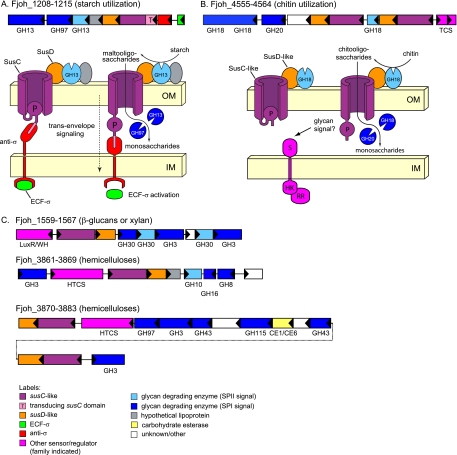
FIG. 2.
Representative PULs of F. johnsoniae. (A) Putative starch utilization PUL with components that are similar to those of the prototypic starch utilization system (Sus) of B. thetaiotaomicron. Like the prototypic Sus system, this system includes two GH13 α-amylases, one of which has a lipidation signal similar to the endo-acting surface enzyme SusG (light blue), and a single GH97 α-glucosidase. Notably, this system lacks a homolog of the inner-membrane-spanning maltose sensor, SusR, and instead is linked to an extracytoplasmic function sigma (ECF-σ)-anti-σ transcriptional regulator pair (green and red, respectively). These regulatory elements function by coupling to a specialized N-terminal “transducing domain” (pink) attached to the SusC-like transporter and together comprise a “transenvelope signaling” pathway spanning both bacterial membranes. (B) Putative chitin utilization PUL. In contrast to the system shown in panel A, this system includes three glycoside hydrolases predicted to target the β-1,4-N-acetylglucosamine linkages found in chitin (GH18 and GH20 enzymes). One of these enzymes has a secretion signal predicted to position it on the cell surface or in the extracellular space (light blue). Unlike other PULs in other bacteroidetes delineated so far, the chitin utilization PUL is associated with a classic two-component regulatory system (dark pink). Other functional labels for each system's schematic diagram are indicated at the bottom. (C) Representative PULs with predicted roles in hemicellulose utilization (see Fig. S2 in the supplemental material for a more complete list). OM, outer membrane; IM, inner membrane; TCS, two-component regulatory system; HTCS, hybrid two-component regulatory system.