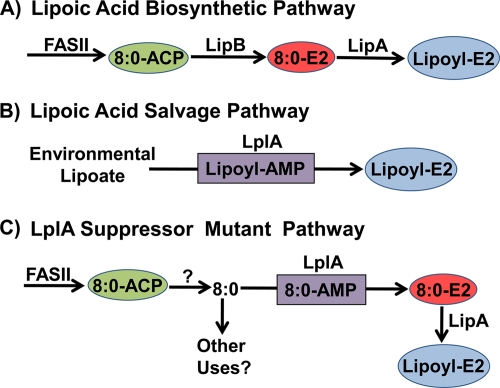
FIG. 2.
Lipoic acid biosynthesis in E. coli. (A) The de novo lipoate biosynthetic pathway begins by the utilization of octanoyl-ACP (8:0-ACP) produced by type II fatty acid synthase (FASII) by LipB to acylate the E2 domain of PDH. Octanoate bound to the E2 domain is converted to lipoic acid by the S-adenylmethionine-dependent LipA protein to form lipoyl-E2. (B) The scavenging pathway utilizes LplA to transfer lipoic acid from the environment to the E2 domain via an acyl-adenylate intermediate. (C) The LplA suppressor pathway is possible due to an intracellular pool of octanoate (8:0) released from the FASII pathway by an unknown thioesterase-like mechanism. Mutant LplA proteins with increased affinity for free octanoate activate and transfer the fatty acid to 8:0-E2. Lipoyl-E2 is then formed by LipA. It is not known whether there are other potential uses for the medium-chain free fatty acid pool in E. coli.