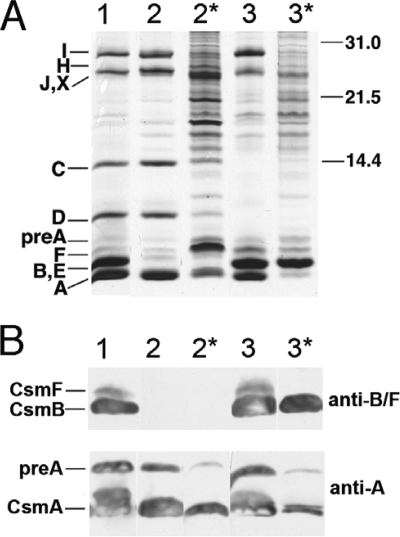
FIG. 1.
SDS-PAGE analysis and immunoblotting of chlorosome fractions from wild-type and mutant strains. (A) Silver-stained SDS-polyacrylamide gels showing the protein composition of chlorosomes and fraction 2 samples. Csm polypeptides are identified at the left, and the migration positions of molecular mass markers (sizes in kDa) are indicated at the right. Lane 1, chlorosomes from the wild type; lane 2, chlorosomes from the csmB csmF mutant; lane 2*, fraction 2 from the csmB csmF mutant; lane 3, chlorosomes from the csmC csmD csmH mutant; lane 3*, fraction 2 from the csmC csmD csmH mutant. Samples containing 2 μg BChl c were loaded for lanes 2* and 3*, and samples containing 10 μg BChl c were loaded for lanes 1, 2, and 3. (B) Immunoblots probed with anti-CsmF and anti-CsmA polyclonal antibodies. Polyclonal antibodies for CsmF cross-react with both CsmF and CsmB. Lane 1, chlorosomes from the wild type; lane 2, chlorosomes from the csmB csmF mutant; lane 2*, fraction 2 from the csmB csmF mutant; lane 3, chlorosomes from the csmC csmD csmH mutant; lane 3*, fraction 2 from the csmC csmD csmH mutant. Samples containing 20 μg of BChl c were loaded for lanes 2* and 3*, and samples containing 100 μg BChl c were loaded for lanes 1, 2, and 3.