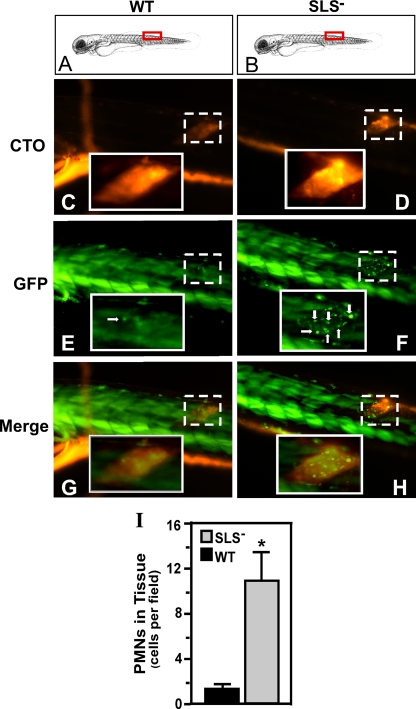
FIG. 4.
The SLS− mutant also promotes a PMN-rich infiltrate in embryos. pu.1 larvae (7 days postfertilization) which express GFP fluorescence specifically in cells of the myeloid lineage were injected in the dorsal muscle with 103 CFU of either the wild type (WT) or the SLS− mutant intrinsically labeled with the fluorescent dye CTO at the site indicated by the red boxes in panels A and B. Infected larvae were examined by fluorescent microscopy at 2 h postinfection for CTO fluorescence (C and D and GFP fluorescence (E and F; PMNs are indicated by white arrows). As noted by Hsu et al., in the larval form, the skeletal muscle (even in wild-type-infected fish) contributes background fluorescence that is easily distinguished from individual myeloid cells (36). The columns show images taken from single representative larvae, oriented as indicated in the top panels (A and B), that were infected with the wild type (WT) or the SLS− mutant. Panels G and H show the merged images. The site of injection in each fish is demarcated in the micrographs by the boxes with the broken white line. Magnification, ×10. The insets outlined by the boxes with the unbroken white lines show the injection site at a higher magnification (×40). The diagram in panels A and B was adapted from Kimmel et al. (39) with permission of the publisher, John Wiley & Sons, Inc. (I) The number of PMNs that infiltrated the infected tissue as determined by microscopic examination by blinded observers. Data shown represent the mean and standard deviation of the number of individual PMNs observed per microscopic field and pooled from three infected zebrafish per experimental group. As with the results of infections of adults, there were significantly more PMNs observed in tissue infected by the SLS− mutant than in wild-type-infected tissue (P < 0.0001).