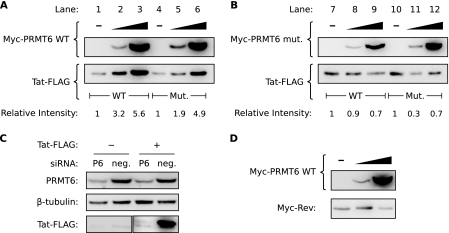
FIG. 1.
Overexpression of catalytically active PRMT6 increases the steady-state level of Tat. (A) HeLa cells were transfected to express wild-type (WT) Tat-FLAG alone (lane 1), wild-type Tat-FLAG with increasing amounts of Myc-PRMT6 (lanes 2 and 3), mutant (Mut.) Tat-R52/53K-FLAG alone (lane 4), or mutant Tat-R52/53K-FLAG with increasing amounts of Myc-PRMT6 (lanes 5 and 6). All samples were cotransfected with a β-galactosidase expression vector, and Western blotting was performed with lysates equalized for β-galactosidase activity. The Tat-FLAG bands were quantified and are expressed relative to wild-type Tat-FLAG alone (lanes 1 to 3) or mutant Tat-R52/53K-FLAG alone (lanes 4 to 6). The blots are representative of six independent experiments. (B) Similar experiments were performed as for panel A but with a catalytically inactive version of Myc-PRMT6 (Myc-PRMT6mut). (C) HeLa cells were transfected with siRNA targeting PRMT6 (P6) or a nontargeting negative control (neg.) and then transfected 24 h later with plasmids expressing wild-type Tat-FLAG (+) or an empty-vector control (−). Cell lysates were Western blotted to confirm PRMT6 knockdown and to assess the effect on Tat steady-state levels. (D) HeLa cells transfected to express Myc-Rev alone (−) or with increasing amounts of wild-type Myc-PRMT6 were Western blotted to determine the effect on Myc-Rev steady-state levels. All samples were cotransfected with β-galactosidase vector, and the lysates were normalized for β-galactosidase activity. Panels B to D are representative of two or three independent experiments.