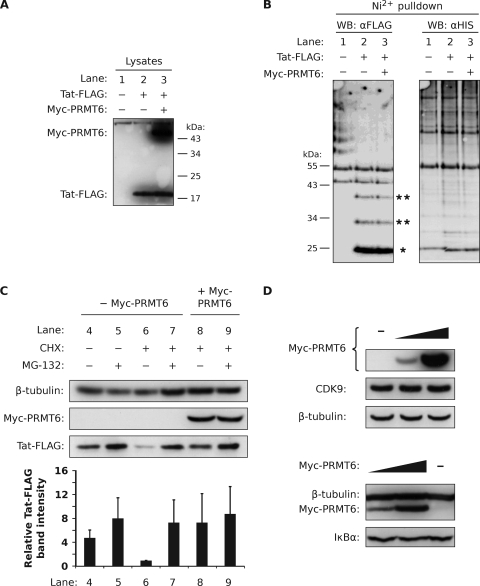
FIG. 5.
Tat is not polyubiquitinated but is degraded by a proteasome. (A) HEK293T cells were transfected with a plasmid expressing His6-Ub alone (lane 1), with Tat-FLAG (lane 2), or with Tat-FLAG and Myc-PRMT6 plasmids (lane 3). Nickel-coated nitrilotriacetic acid-agarose beads were added to lysates equalized for Tat-FLAG protein levels to pull down ubiquitinated conjugates via the His6 tag. (B) Bead eluates were subjected to Western blotting (WB) using the anti-FLAG (αFLAG) antibody (left blot) and the anti-His6 (αHIS) antibody (right blot). The locations of monoubiquitinated Tat-FLAG (single asterisk) and multiply ubiquitinated Tat-FLAG (double asterisks) are indicated. (C) HeLa cells expressing Tat-FLAG with (+) or without (−) Myc-PRMT6 were treated for 3 h with combinations of CHX, the proteasome inhibitor MG-132, or vehicle (DMSO). The cell lysates were then analyzed by Western blotting. The Tat-FLAG bands were quantified and are expressed relative to lane 6, where columns represent mean band intensities and error bars indicate standard deviations. (D) HeLa cells expressing increasing amounts of Myc-PRMT6 were Western blotted for endogenous CDK9 expression (upper blots) and endogenous IκBα expression (lower blots). The β-tubulin levels show equivalent sample loadings. All panels are representative of two or three independent experiments.