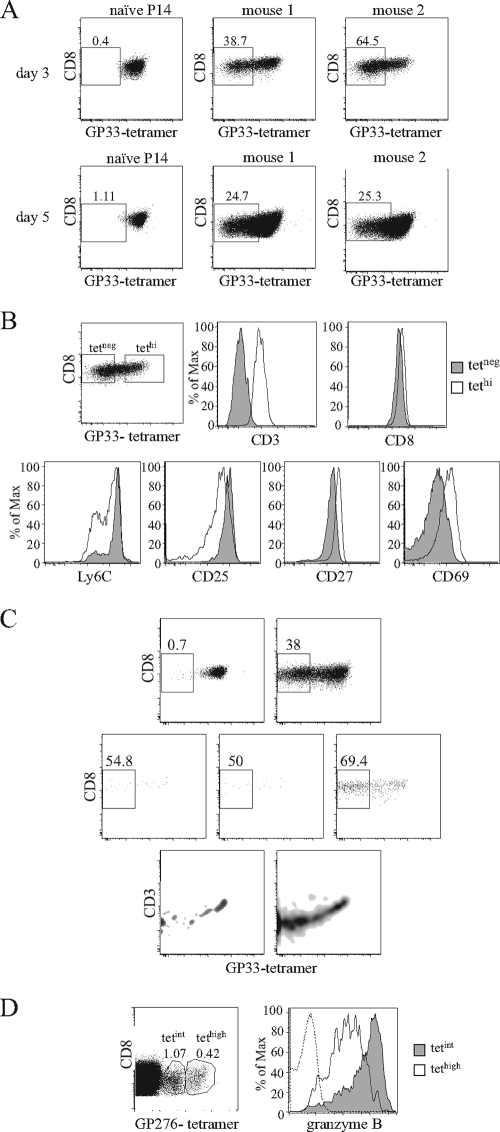
FIG. 5.
MHC-I tetramer labeling during the expansion phase. B6.Ly5.1 mice left untreated or receiving Ly5.2+ P14 Tg cells were infected with 2 × 105 PFU of LCMV Armstrong and studied at different time points after infection. (A and B) Mice were injected with 5 × 105 Tg cells. (A) Results compare GP33 tetramer (tet) binding in Ly5.2+ P14 naïve cells and in P14 cells at on day 3 (top) and day 5 (bottom) after infection. Staining is from individual mice representative of four experiments with two to three mice per time point. (B) On day three after infection, P14 cells were arbitrarily subdivided into tetneg (gray) and tethi (white) subsets, and each population was tested for the expression of the indicated cell surface molecules. Gates for tetneg cells were established in noninfected B6 mice and for tethi in naïve Tg cells. (C) B6 mice were injected with either 5 × 105 or 104 Ly5.2+ P14 Tg cells and studied at days 4 and 5 after infection. Results are for GP33 tetramer binding in P14 Tg cells. Naïve mice (upper left), cells from mice injected with 5 × 105 naïve cells (upper right), and cells from three individual mice injected with 104 naïve cells (lower graphs) are shown. At day 4, very few Tg cells were detected in the latter mice. (D) Granzyme B expression in cells expressing different tet binding intensity in normal mice. CD8 cells were recovered 5 days after infection, labeled with GP276 tetramers, and subdivided into tetint and tethi populations. Results show the gates used for such subdivision and intracellular granzyme B staining for tetint (gray) and tethi (white) populations; the dashed lines represent the staining of the same cells with an isotype control antibody.