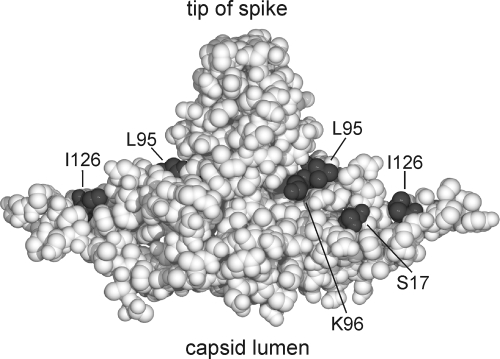
FIG. 3.
Positions of aa residues in the core protein homodimer that are relevant for capsid envelopment. A sphere model of the core protein homodimer is shown. Even conservative point mutations at aa residues S17, K96, and I126 and less conservative mutations at L95, exposed at the outer capsid surface, blocked virion formation (Fig. 2A). We assume that they form an area for the interaction with cellular or viral factors (envelope proteins) required for nucleocapsid envelopment.