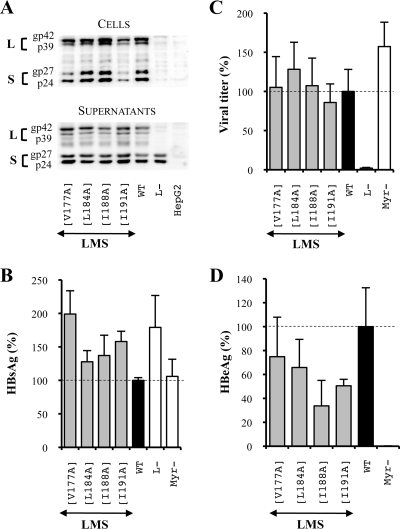
FIG. 7.
The unique substitution of amino acids constitutive of the TM1 domain heptad repeat does not affect the HBV viral cycle. LMS means that mutations were introduced in the three envelope proteins. For this purpose, the mutagenesis was performed in the pSV12SX and pSVSX plasmids. For the three graphics, values were expressed as percentages of the WT condition, and sample standard deviations were determined by the analysis of three sets of experiments. The horizontal dotted lines indicate the level of the WT condition. L− and Myr− were used as controls. (A) Analysis of intra- and extracellular mutated protein expression. Cellular proteins (cells), harvested 12 days posttransfection, were probed with an anti-S antibody (1:1,000). Pools of culture supernatants that were collected between days 6 and 12 posttransfection were precipitated with 8% PEG, disrupted in sample buffer (Invitrogen), and analyzed as described above. HepG2 and L− were used as negative controls. Molecular sizes of glycosylated (gp) and unglycosylated (p) HBV envelope proteins are indicated. (B) HBsAg secretion. The effect of mutations on the secretion ability of envelope proteins was assessed by measuring the HBsAg level in pools of culture supernatants, collected between days 6 and 12 posttransfection, of HepG2 cells producing virions. The HBsAg concentration was determined with a commercial ELISA (Bio-Rad). (C) Virus titers. The number of complete particles in inocula was determined by Q-PCR analysis of viral DNA extracted from immuno-captured virus. (D) Infectivity. Infection was assessed by measuring the HBeAg level in the culture supernatant of infected HepaRG cells 10 days postinfection with a commercial ELISA (Bio-Rad). The infectivity was expressed as a ratio between the level of HBeAg and the number of GEq used for infection.