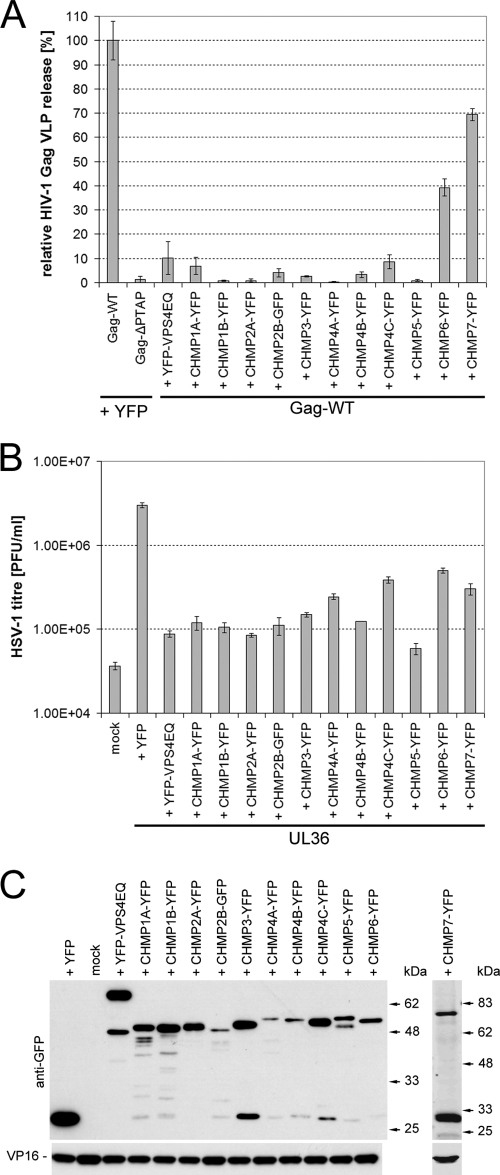
FIG. 3.
Dominant-negative ESCRT-III proteins potently inhibit the production of infectious HSV-1. (A) HIV-1 Gag VLP release assays were performed to test the ability of dominant-negative CHMPs to block an ESCRT-mediated virus budding process. 293T cells were cotransfected with a plasmid expressing YFP-tagged HIV-1 Gag together with a plasmid encoding YFP/GFP-tagged CHMPs, YFP-VPS4EQ or YFP. Cells cotransfected with YFP-Gag-ΔPTAP- and YFP-expressing plasmids served as a negative control since little VLP release is expected in the absence of the PTAP late-domain motif. Cell culture medium samples from three parallel samples of each condition were harvested 24 h posttransfection and clarified by low-speed centrifugation, and then VLPs were harvested by high-speed centrifugation (30,000 rpm, 4°C, 60 min; TLA-55 rotor). Samples were analyzed by Western blotting, and the amount of HIV-1 Gag present in each sample was quantified by densitometry analysis of scanned blots using ImageJ. The data were normalized to the mean level of YFP-tagged HIV-1 Gag released in the presence of YFP. Bars represent the mean VLP release, and error bars indicate the standard errors of the mean of triplicate samples. (B) COS-7 cells were cotransfected with a plasmid expressing the essential HSV-1 UL36 gene product, together with a plasmid encoding YFP/GFP-tagged CHMPs, YFP-VPS4EQ, or YFP. Mock-treated cells (mock) did not receive any plasmid DNA. Transfected cells were infected with HSV-1 lacking the UL36 gene (KΔUL36), which will only produce infectious virions in UL36 expressing cells. Infected cells were scraped and sonicated to release infectious progeny virus and HSV-1 titers were determined by plaque assay on a UL36 complementing cell line (HS30). Bars represent mean PFU per ml, and error bars indicate the standard errors of the mean of triplicate samples. (C) Protein samples collected from each condition were analyzed by Western blotting to demonstrate the expression levels of the YFP-tagged proteins, as well as the viral tegument protein VP16.