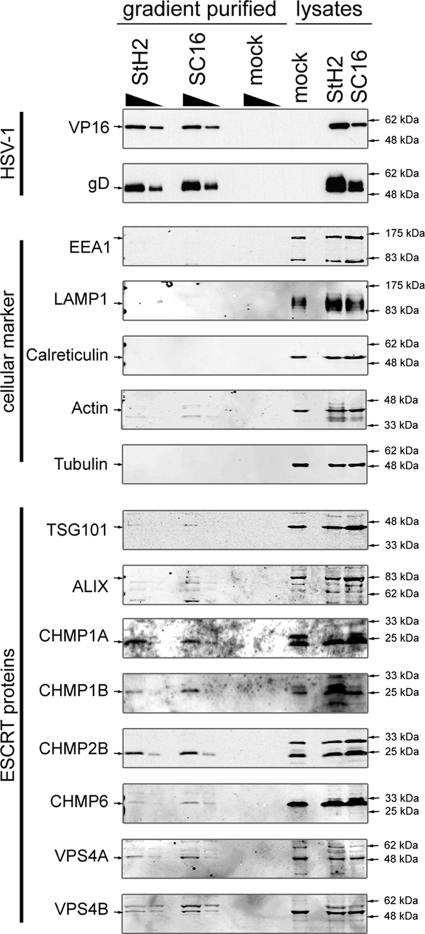
FIG. 6.
ESCRT proteins are associated with purified HSV-1 virions. HaCaT cells were grown in roller bottles until confluent and then infected either with the HSV-1 strains SC16 or StH2 at 0.1 PFU/cell or with medium without virus (mock). Cell culture medium was harvested at 48 h postinfection and clarified of cell debris by two subsequent low-speed centrifugation steps (2,900 rpm, 10 min, 4°C; Beckman GH-3.8). After concentration by high-speed centrifugation (18,000 rpm, 4°C, 90 min; type 19 rotor), virus particles were purified on a 5 to 15% Ficoll continuous gradient (12,000 rpm, 4°C. 90 min; SW 32Ti rotor). The appropriate density light scattering band corresponding to mature, infectious HSV-1 particles was collected by side puncture. The same volume of mock sample at the same level in the gradient as the light scattering band was harvested. After determination of the infectious virus titers on Vero cells, 1.5 × 108 PFU and 5 × 107 PFU of each virus preparation and an identical volume of the mock sample were analyzed by Western blotting with specific antibodies to markers of cellular compartments and ESCRT proteins. Then, 1.5 × 107 PFU and 5 × 106 PFU of each virus preparation and an identical volume of the mock sample were analyzed by Western blotting with specific antibodies to the viral proteins VP16 and gD.