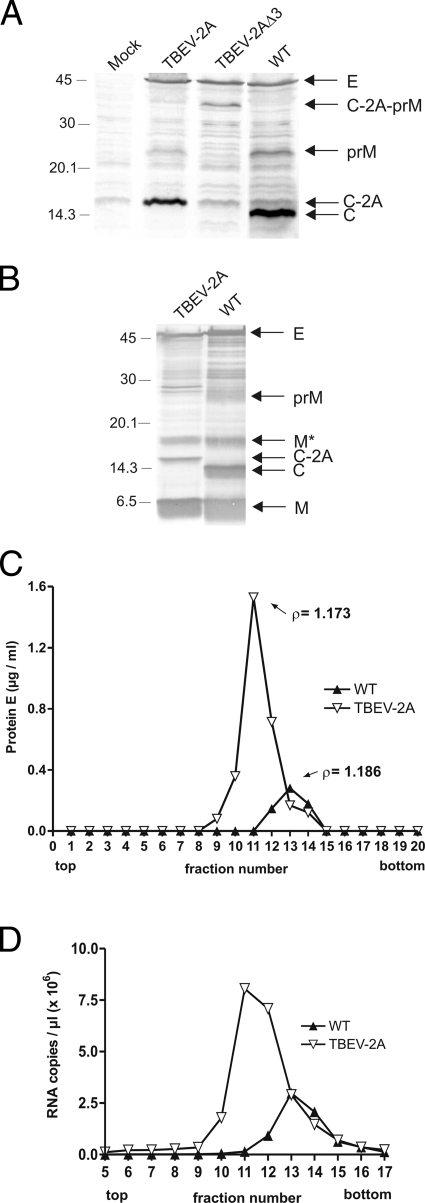
FIG. 2.
Analysis of structural-protein processing (A) and secreted particles (B to D). (A) Cells transfected with WT or mutant RNAs were lysed 24 h posttransfection, and proteins were detected by immunoblotting using a rabbit polyclonal serum against the TBEV structural proteins. (B) Cells were transfected with TBEV-2A RNA; 48 h posttransfection, the components of particles secreted from the cells were compared to those of a WT preparation by immunoblotting using the same antiserum as in panel A. The WT preparation was selected because it contained an unusually high percentage (15%) of immature virus and thus a substantial amount of prM, enabling the identification of the protein. The positions of the structural proteins E, prM, M, C, and C-2A and the uncleaved precursor protein C-2A-prM are marked on the right those of marker proteins in kDa on the left. The M* band is thought to be an “M-dimer,” as described elsewhere, as it was previously shown by direct amino-terminal sequence analysis to contain protein M (37). (Panels A and B were edited with Adobe Photoshop to change the order of the lanes and to exclude unrelated samples.) (C and D) Cells were transfected with TBEV-2A RNA; 48 h posttransfection, particles were subjected to rate zonal and equilibrium density ultracentrifugation, and their protein E (C) and RNA (D) contents were determined as described in Materials and Methods. Virus purified by the same ultracentrifugation protocol was used as a control. The buoyant densities of the particles are indicated in panel C.