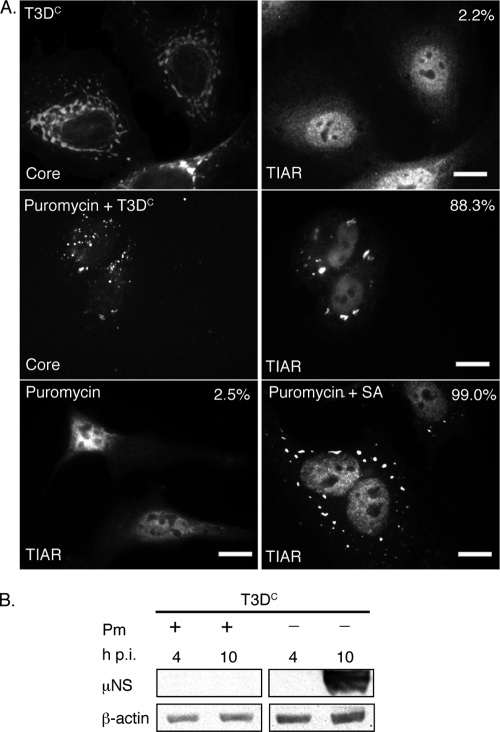
FIG. 6.
Puromycin inhibits viral translation but does not prevent MRV induction of SGs. (A) HeLa cells were untreated (first row) or pretreated with 0.1 mg/ml puromycin (second row) for 1 h and then infected with MRV T3DC virions (1,000 PFU/cell) for 1 h. New medium containing 0.1 mg/ml puromycin was added to cells following infection (second row). At 10 h p.i., cells were fixed and immunostained with rabbit anti-MRV core polyclonal antisera (first and second rows, left column) and goat anti-TIAR polyclonal antibodies (first and second rows, right column), followed by Alexa 594-conjugated donkey anti-rabbit IgG and Alexa 488-conjugated donkey anti-goat IgG. Uninfected HeLa cells were treated with 0.1 mg/ml puromycin (third row, left and right columns), incubated (left column) or treated with 0.5 mM SA for 1 h (right column) after 9 h puromycin treatment, and then fixed and stained with goat anti-TIAR polyclonal antibodies followed by Alexa 488-conjugated donkey anti-goat IgG. Following immunostaining, the percentage of infected cells containing SGs (first and second rows) or total cells containing SGs (third row) was quantified as described in Materials and Methods. Percentages of SG-containing cells are indicated. Scale bars = 10 μm. (B) HeLa cells were infected with MRV T3DC virions (1,000 PFU/cell) with or without 0.1 mg/ml puromycin (Pm) as indicated, and at 4 and 10 h p.i., cells were lysed, and proteins were separated by SDS-PAGE, followed by immunoblotting using anti-μNS polyclonal antiserum or anti-β-actin polyclonal antibodies. Proteins were detected using HRP-conjugated goat anti-rabbit IgG, followed by chemiluminescence imaging.