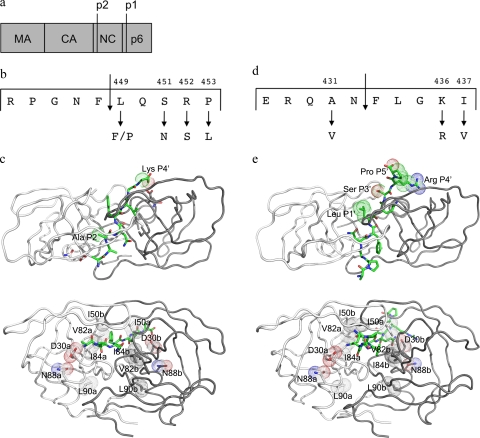
FIG. 1.
(a) Overview of Gag showing the constituent proteins. (b) Details of the NC-p1 cleavage site. Also shown are two residues that mutate frequently. (c) Two different views of the crystal structure of HIV-1 protease complexed with NC-p1 cleavage site (1TSU) (33). The substrate is shown in green, and the two monomers are shown in white and gray. (Top) The top view showing van der Waals surfaces on cleavage site residues that mutate frequently. (Bottom) The side view with van der Waals surfaces on protease residues that are the focus of this study. (d) Details of the p1-p6 cleavage site. Frequently occurring mutations are shown. (e) Two different views of the crystal structure of HIV-1 protease complexed with p1-p6 cleavage site (1KJF) (34). Shown are the substrate (green) and the two monomers (white and gray). (Top) The top view showing van der Waals surfaces on cleavage site residues that mutate frequently. (Bottom) The side view with van der Waals surfaces on protease residues that are the focus of this study. Images were generated using PyMol (8).